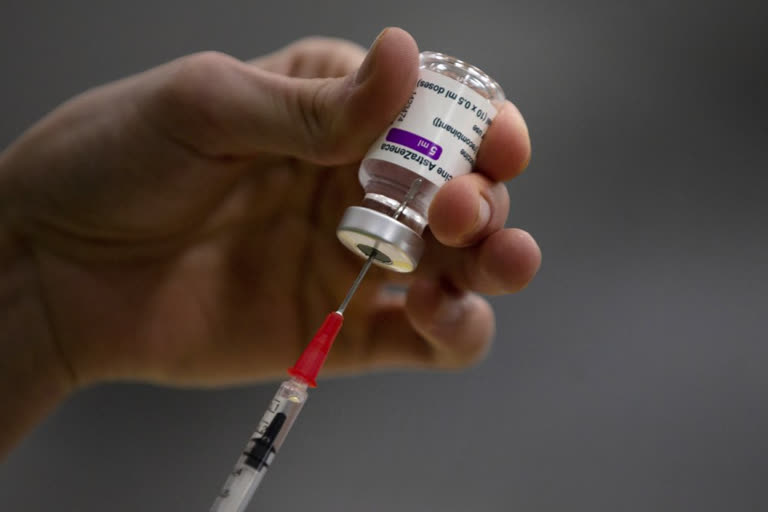
Moscow: The Dutch authorities are halting the use of the AstraZeneca vaccine against the coronavirus amid concerns over recent blood clotting incidents.
"On the basis of new information, the Dutch Medicines Evaluation Board (CBG) has advised, as a precautionary measure and pending further investigation, to pause the administration of the AstraZeneca corona vaccine," the Dutch government announced on Sunday.
Read: Britain will receive 10 mln doses of AstraZeneca vaccine from SII
According to the statement, the AstraZeneca vaccine will not be used in the next two weeks, until March 28.
Earlier on Sunday, Ireland suspended the use of the AstraZeneca vaccine as new information emerged on blood clotting incidents in Norway.
Read: 'No indication Oxford vax linked to blood clots'
On Saturday, the Norwegian Medicines Agency said that more cases of blood clotting had been found in adults who received AstraZeneca vaccine shots.
Earlier this week, several European countries, including Denmark, Iceland and Norway, halted the rollout of the AstraZeneca vaccine after Austria said it was investigating the death of a vaccinated woman from multiple thromboses.
Read: Vaccine Against New COVID Variants May Take 6-9 Months: AstraZeneca
The UK-Swedish pharmaceutical giant AstraZeneca said on Sunday that it had found no evidence of increased risk of blood clots from its COVID-19 vaccine after conducting a review of safety data of over 17 million inoculated people across the European Union and the United Kingdom.
ANI