Hyderabad: COVAXINTM, India's indigenous COVID-19 vaccine is developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) - National Institute of Virology (NIV).
The indigenous, inactivated vaccine is developed and manufactured in Bharat Biotech's BSL-3 (Bio-Safety Level 3) high containment facility.
After successful completion of the interim analysis from the Phase 1 & 2 clinical trials of COVAXINTM, Bharat Biotech received DCGI approval for Phase 3 clinical trials in 26,000 participants in over 25 centres across India.
What is ‘Covaxin’ and how was it developed
The vaccine candidate was developed by BBIL in collaboration with the National Institute of Virology (NIV). The SARS-CoV-2 strain was isolated in NIV, Pune and transferred to Bharat Biotech. The indigenous, inactivated vaccine developed and manufactured in Bharat Biotech’s BSL-3 (Bio-SafetyLevel 3) High Containment facility located in Genome Valley, Hyderabad, India.
Bharat Biotech is the only vaccine company in the world which has BSL 3 production facility (Biosafety level 3).
Collaboration with US company
Bharat Biotech has currently invested in two other vaccines: CoroFlu in collaboration with FluGen Inc. and the University of Wisconsin-Madison, and an inactivated rabies vaccine vehicle for coronavirus proteins developed along with Matthis Schnell, director of the Jefferson Vaccine Centre (JVC), Pennsylvania. The latter is of interest.
On May 20, Bharat Biotech announced its collaboration with JVC as well as the license it had received to conduct clinical trials and to produce and deliver vaccines in 80 countries excluding the US, Europe and Japan. On April 7, JVC announced a promising vaccine candidate named Coravax.
Coravax uses an inactivated rabies vaccine to carry the spike protein of the novel coronavirus. The spike protein attaches to a host cell and causes an infection, so experts expected this vaccine to trigger a good immune response on the body’s part. Schnell corroborated this response following preliminary tests with animals. Schnell added that JVC would need one more month to complete follow-up studies.
Using a rabies vaccine to deliver a vaccine for the novel coronavirus is a bit of technology that researchers perfected for use against the MERS and SARS viruses as well. And it’s possible that Bharat Biotech’s Covaxin uses the same technology.
In early 2019, Bharat Biotech acquired Chiron Behring Vaccines Pvt. Ltd. from GlaxoSmithKline and ramped up production of the rabies vaccine Chirorab to 15 million units a year. So the company already has the ability to mass-produce this vaccine.
Status of Clinical trials
Bharat Biotech, along with the Indian Council of Medical Research is developing India's first home-grown vaccine. Known by the name Covaxin, the vaccine candidate is currently going through phase 3 trials at several locations, including AIIMS Delhi. According to recent reports, the company said that they aim at least 60 per cent efficacy for the vaccine, after which it is expected to be rolled out mid of 2021.
The third trial may be completed by early 2021. The most important thing is that India works on an affordable biotech vaccine, so it is expected that the Covaxin may be the cheapest vaccine in the world.
According to another report, Bharat Biotech will manufacture the COVID-19 vaccine Covaxin, in Odisha, along with nine other vaccines.
Status of Indian-made COVID-19 vaccines
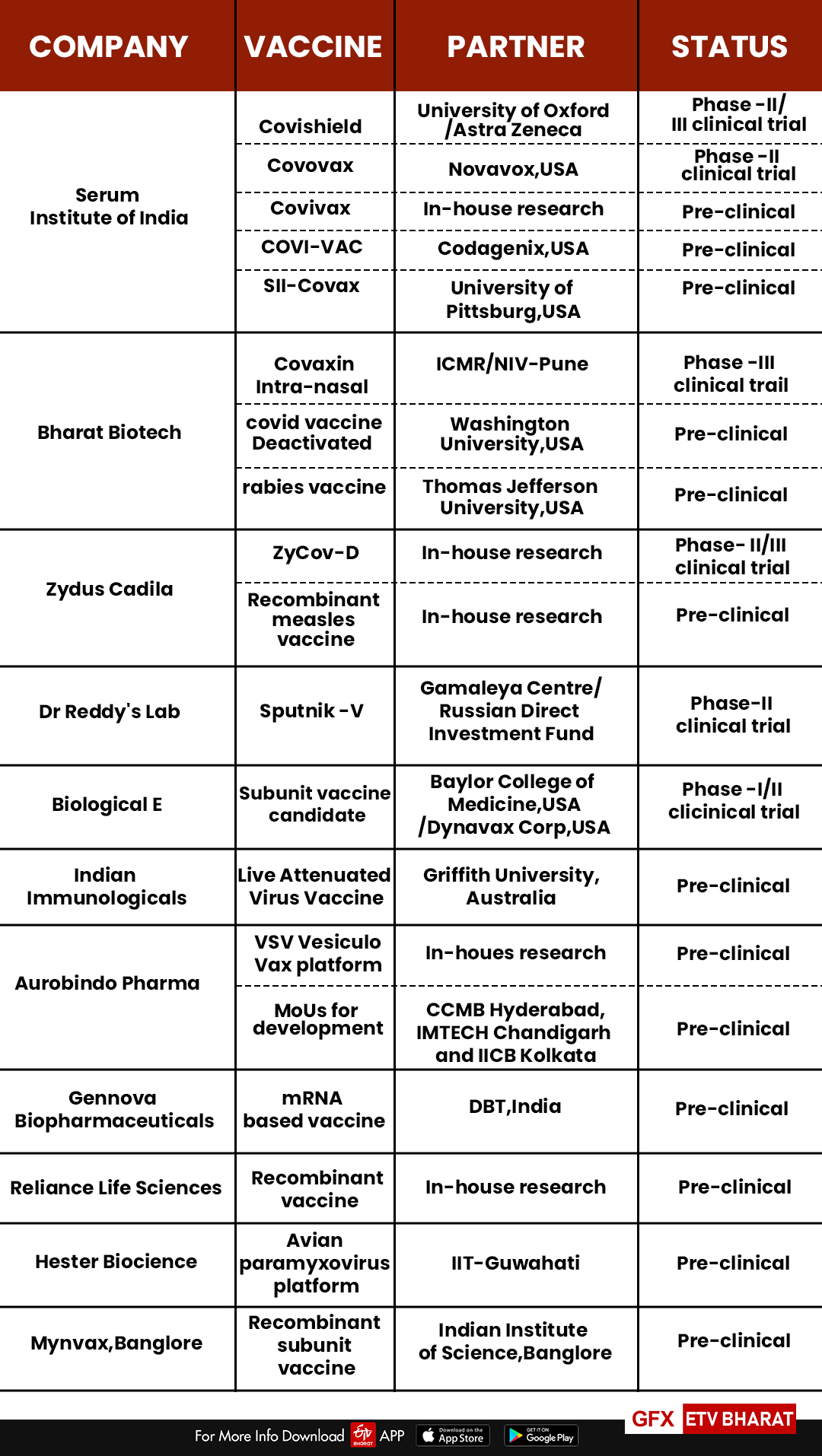
Currently,11 Indian companies working towards COVID-19 vaccine development. Thereby they have joined the global endeavour to find a quick preventive measure for the fatal virus infection. And it needs to be faster to prevent the virus from spreading rapidly across the world.
About Bharat Biotech
Bharat Biotech has established an excellent track record of innovation with more than 140 global patents, a wide product portfolio of more than 16 vaccines, 4 bio-therapeutics, registrations in more than 116 countries and WHO Pre-qualifications. Located in Genome Valley, the hub for the global biotech industry, the company has built a world-class vaccine & bio-therapeutics, research & product development, Bio-Safety Level 3 manufacturing, and vaccine supply and distribution.
Having delivered more than 4 billion doses of vaccines worldwide, Bharat Biotech continues to lead innovation and has developed vaccines for H1N1, Rotavirus, Japanese Encephalitis, Rabies, Chikungunya, Zika and the world’s first conjugated vaccine for Typhoid.
The company is proficient in conducting extensive multi-centre clinical trials, having completed more than 75 trials in over 300,000 subjects globally. Our commitment to global social innovation programs and public-private partnerships resulted in the introduction of path-breaking WHO pre-qualified vaccines BIOPOLIO® ROTAVAC® and Typbar TCV® combatting Polio, Rotavirus and Typhoid infections respectively. Bharat Biotech has successfully partnered with NIV-ICMR having developed JENVAC®, a licensed Japanese Encephalitis vaccine. The recent acquisition of the Rabies vaccine facility, Chiron Behring, Ankleshwar has positioned Bharat Biotech as the largest Rabies vaccine manufacturer in the world.