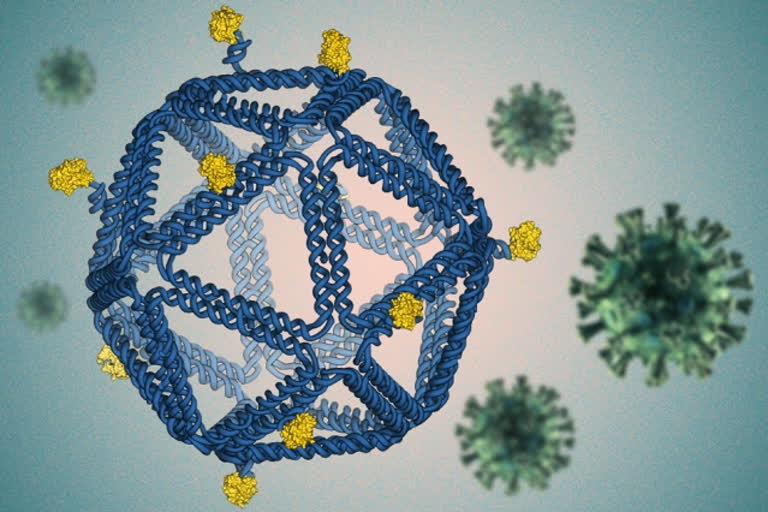
Hyderabad: Researchers from the Massachusetts Institute of Technology (MIT) have designed HIV-like particles by folding DNA into a virus-like structure. These particles provoke a strong immune response from human immune cells grown in a lab dish using a technique called DNA origami and may prove useful in developing an HIV vaccine.
The DNA particles, which closely mimic the size and shape of viruses, are coated with HIV proteins, or antigens, arranged in precise patterns designed to provoke a strong immune response.
The researchers are now working on adapting this approach to develop a potential vaccine for SARS-CoV-2 or Covid-19, and they anticipate it could work for a wide variety of viral diseases.
“The rough design rules that are starting to come out of this work should be generically applicable across disease antigens and diseases,” says Darrell Irvine, who is the Underwood-Prescott Professor with appointments in the departments of Biological Engineering and Materials Science and Engineering.
The study was published in Nature Nanotechnology - a monthly peer-reviewed scientific journal published by Nature Publishing Group.
Irvine and Mark Bathe, who is an MIT professor of biological engineering and an associate member of the Broad Institute of MIT and Harvard, are the senior authors of the study, while the paper’s lead authors are former MIT postdocs Rémi Veneziano and Tyson Moyer.
DNA origami
Scientists have been working since the 1980s on methods to design DNA molecules that could be used for drug delivery and many other applications, and most recently using a technique called DNA origami that was invented in 2006 by Paul Rothemund of Caltech.
In 2016, Bathe’s lab developed an algorithm that can automatically design and build arbitrary three-dimensional virus-like shapes using DNA origami.
This method offers precise control over the structure of synthetic DNA, allowing researchers to attach a variety of molecules, such as viral antigens, at specific locations.
“The DNA structure is like a pegboard where the antigens can be attached at any position,” Bathe says. “These virus-like particles have now enabled us to reveal fundamental molecular principles of immune cell recognition for the first time.”
Natural viruses are nanoparticles with antigens arrayed on the particle surface, and it is thought that the immune system (especially B cells) has evolved to efficiently recognize such particulate antigens. Vaccines are now being developed to mimic natural viral structures, and such nanoparticle vaccines are believed to be very effective at producing a B cell immune response because they are the right size to be carried to the lymphatic vessels, which send them directly to B cells waiting in the lymph nodes. The particles are also the right size to interact with B cells and can present a dense array of viral particles.
Also read: Sheila Irene Pant: Daughter of Almora who became 'Madar-e-Watan' of Pakistan
However, determining the right particle size, the spacing between antigens, and a number of antigens per particle to optimally stimulate B cells (which bind to target antigens through their B cell receptors) has been a challenge. Bathe and Irvine set out to use these DNA scaffolds to mimic such viral and vaccine particle structures, in hopes of discovering the best particle designs for B cell activation.
“There is a lot of interest in the use of virus-like particle structures, where you take a vaccine antigen and array it on the surface of a particle, to drive optimal B-cell responses,” Irvine says. “However, the rules for how to design that display are really not well-understood.”
Other researchers have tried to create subunit vaccines using other kinds of synthetic particles, such as polymers, liposomes, or self-assembling proteins, but with those materials, it is not possible to control the placement of viral proteins as precisely as with DNA origami.
For this study, the researchers designed icosahedral particles with a similar size and shape as a typical virus. They attached an engineered HIV antigen related to the gp120 protein to the scaffold at a variety of distances and densities. To their surprise, they found that the vaccines that produced the strongest response B cell responses were not necessarily those that packed the antigens as closely as possible on the scaffold surface.
“It is often assumed that the higher the antigen density, the better, with the idea that bringing B cell receptors as close together as possible is what drives signalling. However, the experimental result, which was very clear, was that actually, the closest possible spacing we could make was not the best. And, and as you widen the distance between two antigens, signalling increased,” Irvine says.
The findings from this study have the potential to guide HIV vaccine development, as the HIV antigen used in these studies is currently being tested in a clinical trial in humans, using a protein nanoparticle scaffold.
Based on their data, the MIT researchers worked with Jayajit Das, a professor of immunology and microbiology at Ohio State University, to develop a model to explain why greater distances between antigens produce better results. When antigens bind to receptors on the surface of B cells, the activated receptors crosslink with each other inside the cell, enhancing their response. However, the model suggests that if the antigens are too close together, this response is diminished.