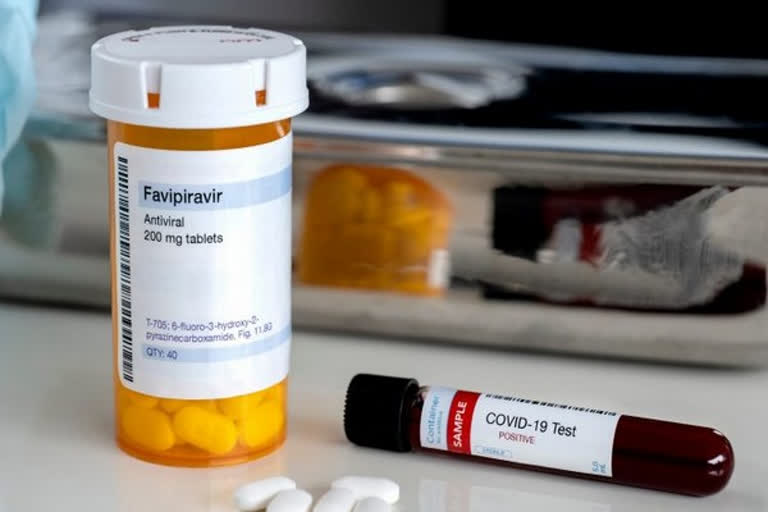
New Delhi: Glenmark Pharmaceuticals on Tuesday said it has initiated Phase 3 clinical trials on antiviral drug Favipiravir to check its efficacy on COVID-19 patients in India.
The Mumbai-based company had received approval from Drug Controller General of India (DCGI) last month to conduct clinical trials of Favipiravir antiviral tablets for the treatment of COVID-19 patients.
Glenmark Pharmaceuticals is the first company in the country to initiate Phase 3 clinical trials on Favipiravir for COVID-19 patients in India, it said in a statement.
Clinical trials have commenced and over ten leading government and private hospitals are being enrolled for the study, it added.
Glenmark estimates study completion by July/August 2020, it said.
As per the approved clinical trial protocol, 150 subjects with mild to moderate COVID-19 symptoms will be randomised in the study in a 1:1 ratio to Favipiravir with standard supportive care or standalone standard supportive care.
Treatment duration is a maximum of 14 days and the total study duration will be a maximum of 28 days from randomisation.
Read more:Over 45,000 bookings worth Rs 16 crore so far for special trains: Railways
"Several health and medical experts, both in and outside of Glenmark are eager to see the effect that Favipiravir has on COVID-19 cases. We believe the study results will be significant as there is currently no effective treatment for the virus," Glenmark Pharmaceuticals Vice President & Head Clinical Development, Global Specialty/Branded Portfolio Monika Tandon said.
The data we get from these trials will point us in a clearer direction with regard to COVID-19 treatment and management, she added.
The company said it aims to launch a treatment for COVID-19 patients as soon as possible and control the spread of the pandemic.
Favipiravir, which is a generic version of Japan-based Fuji film Toyama Chemical's Avigan, has demonstrated activity against influenza viruses and has been approved in Japan for the treatment of novel influenza virus infections.
(PTI Report)