New Delhi: As many as 111 drugs manufactured by different pharmaceutical companies have been found as not of standard quality (NSQ) by the Central Drugs Laboratories as well as State Drugs Testing Laboratories in November.
“The action is being taken by the drug regulators to ensure that these drugs are identified and removed from the market,” the health ministry said on Friday.
The central drugs laboratories have identified 41 drug samples to be not of standard quality and state drugs testing laboratories have identified 70 drug samples as not of standard quality in November, the ministry said.
Glimepride, Pioglitazone Hydrochloride & Metformin Hydrochloride (Extended Release) Tablets (OZOMET-PG 2) manufactured by M/s. Ozone Pharmaceuticals of Solan district in Himachal Pradesh helps to control blood sugar levels, Sucralfate USP for ulcer treatment is manufactured by M/s. Par Drugs & Chemicals Limited of Bhavnagar in Gujarat, Promethazine Injection IP 25 mg/ml manufactured by M/s. Ciron Drugs & Pharmaceuticals Pvt. of Palghar in Maharashtra is used for treating allergies, Aspirin Gastro-resistant Tablets IP 75 mg manufactured by M/s. Unicure India Ltd. of Gautam Budh Nagar in Uttar Pradesh among others have been found as not of standard quality.
Pantoprazole Tablets IP (Opipan -40) manufactured by M/s. Orison Pharma International of Sirmour in Himachal Pradesh which helps in the relief of acid-related indigestion and heartburn has also been found by Central Drugs Laboratories as NSQ.
Similarly, Zylpan is used in the treatment of Acidity, Gastroesophageal reflux disease (Acid reflux) and, Peptic ulcer disease and is manufactured by Assam-based Hetero Healthcare Ltd., Paracetamol Tablets IP 500mg that is used to treat pain (including headache, toothache, back and period pain) and cold or flu symptoms and manufactured by M/s.Troikaa Pharmaceuticals Ltd. of Gujarat has also been found by the State Drugs Testing Laboratories as not of standard quality.
Here is the list of 41 substandard drug samples
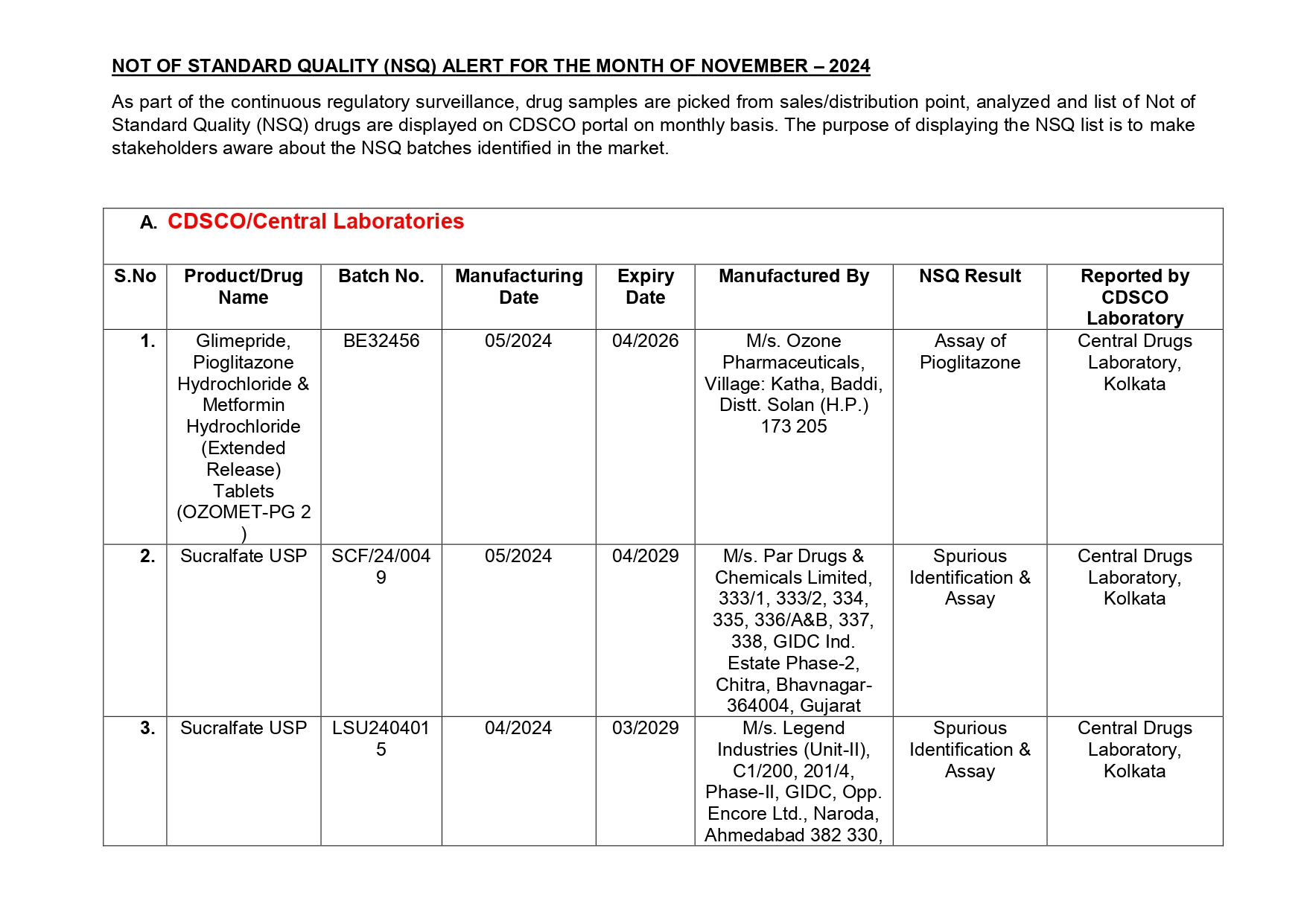
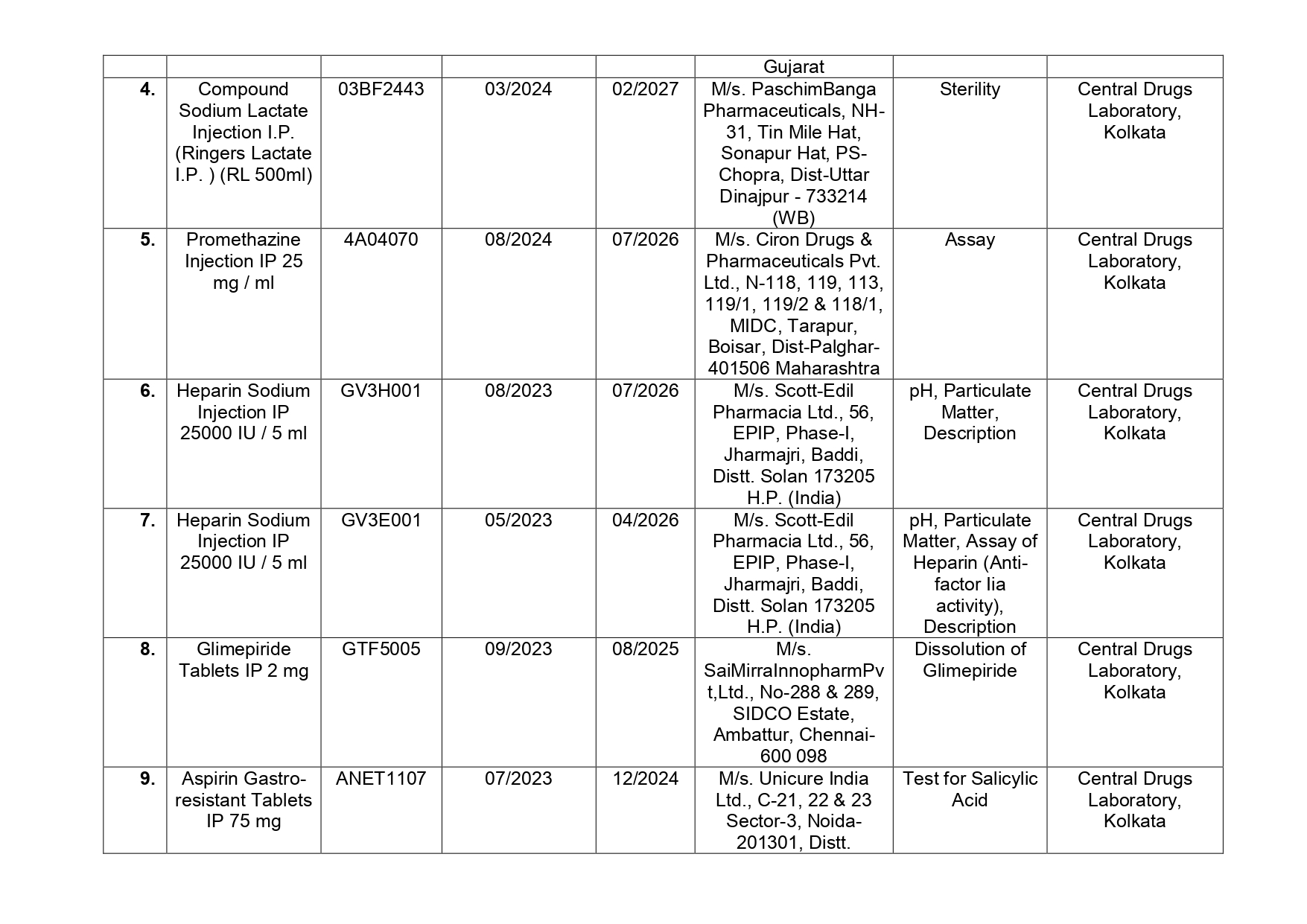
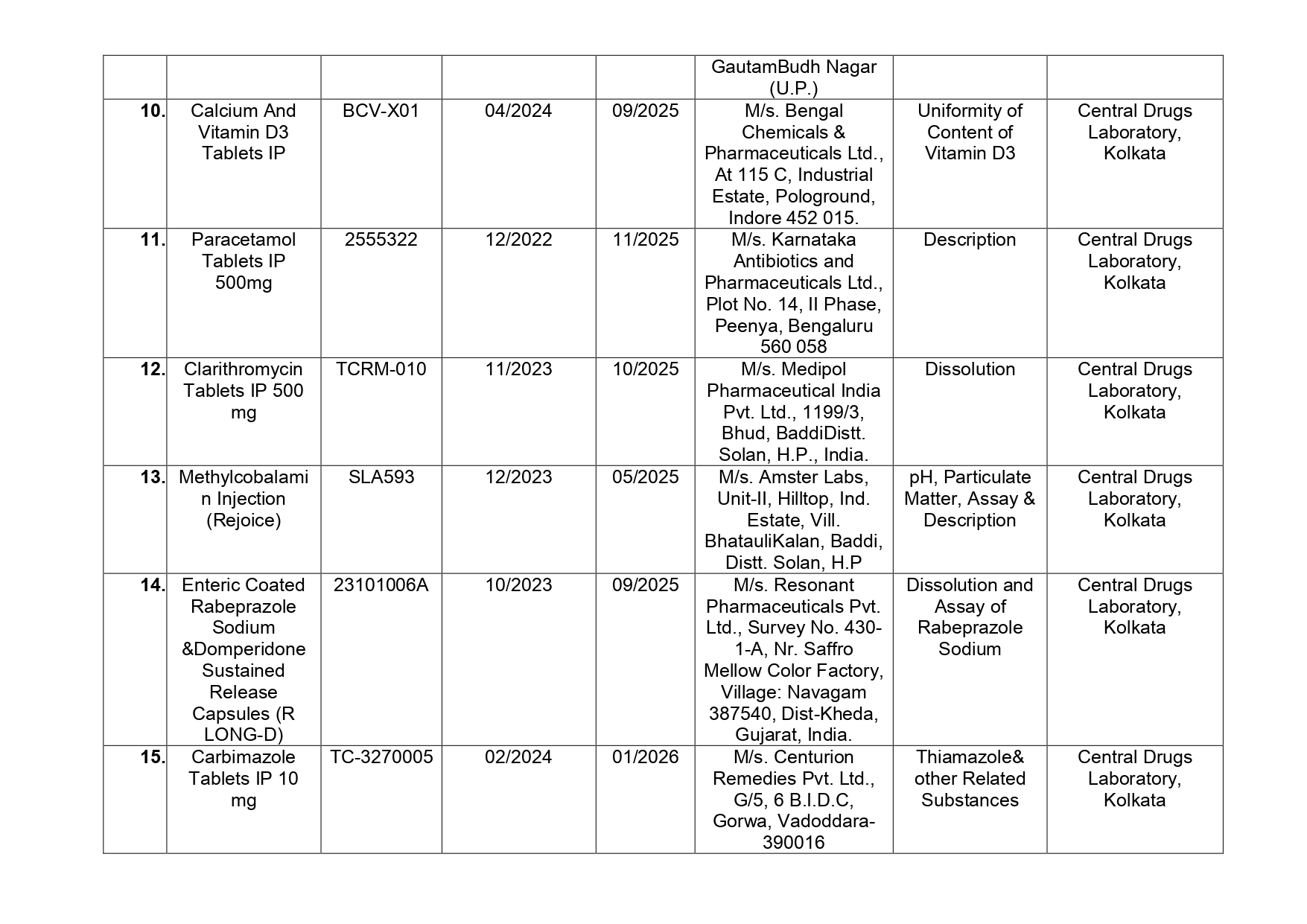
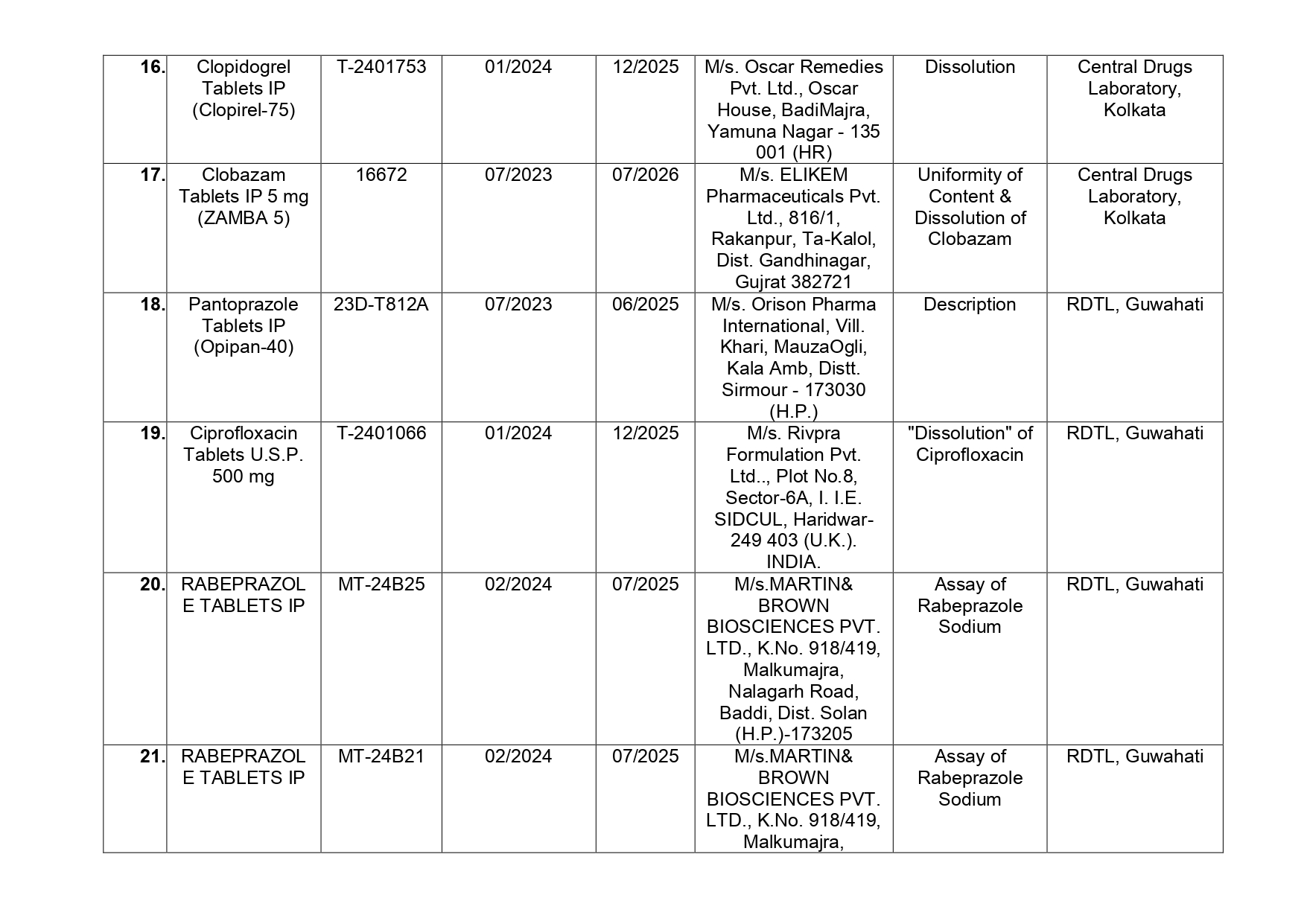
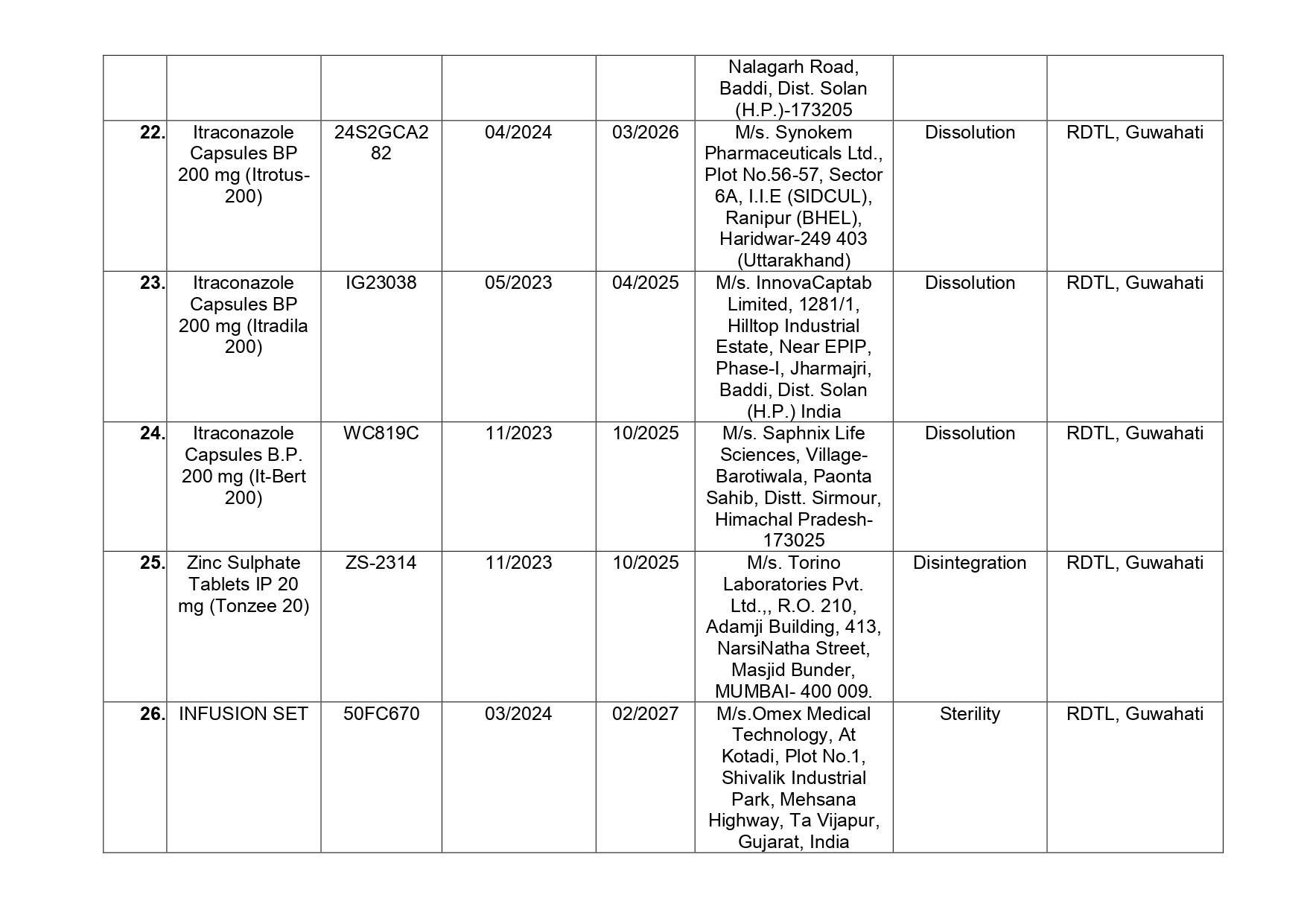
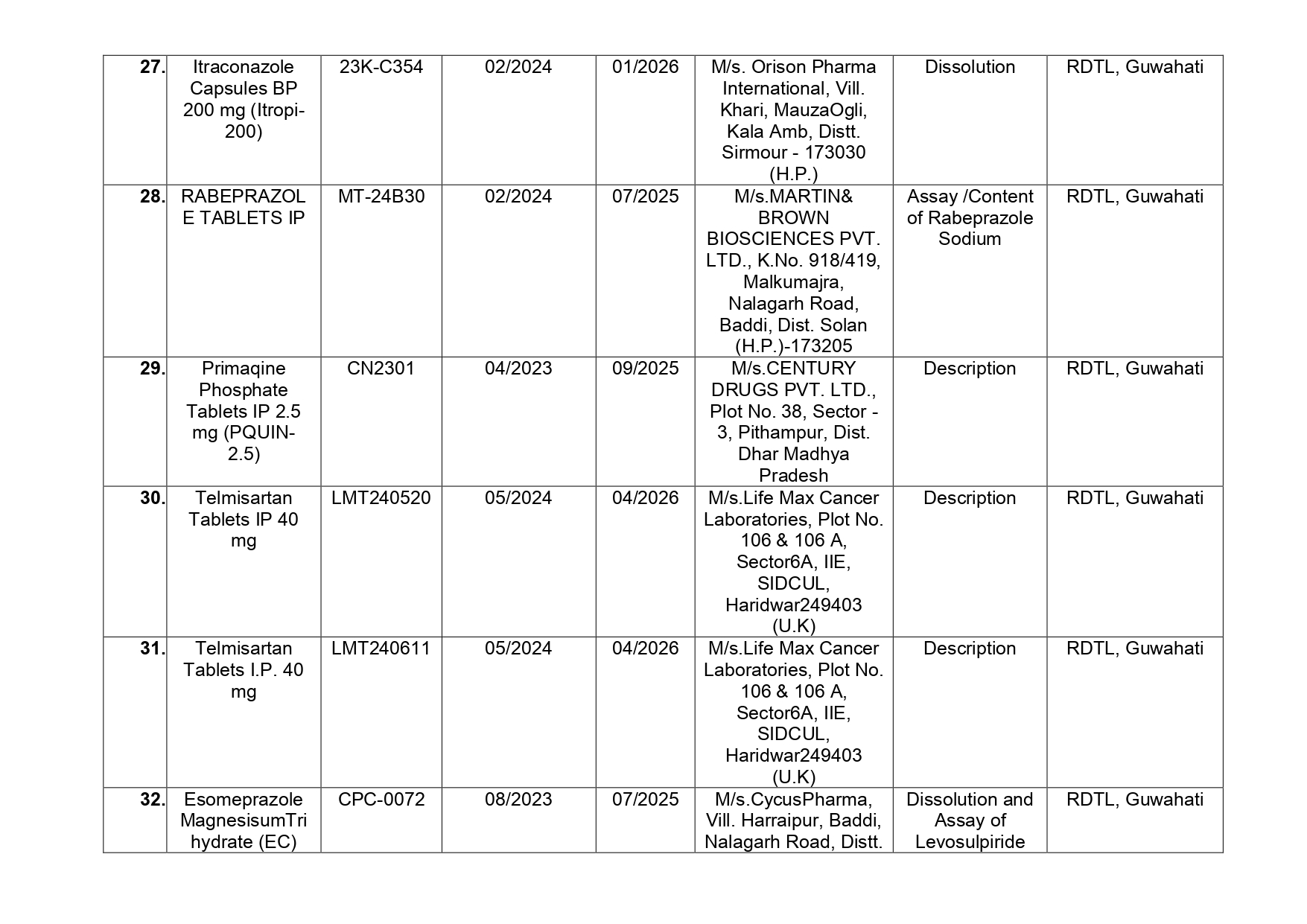
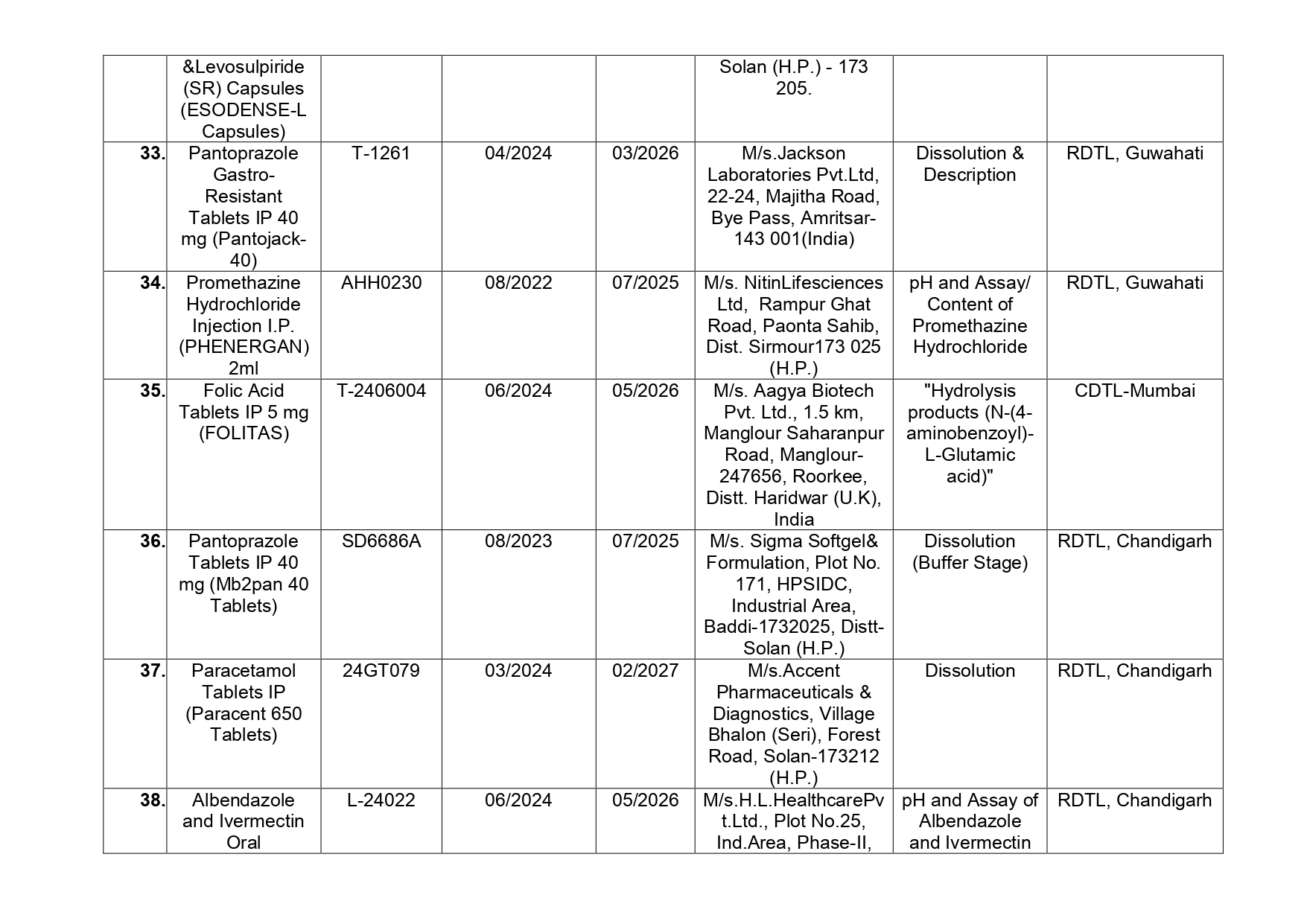
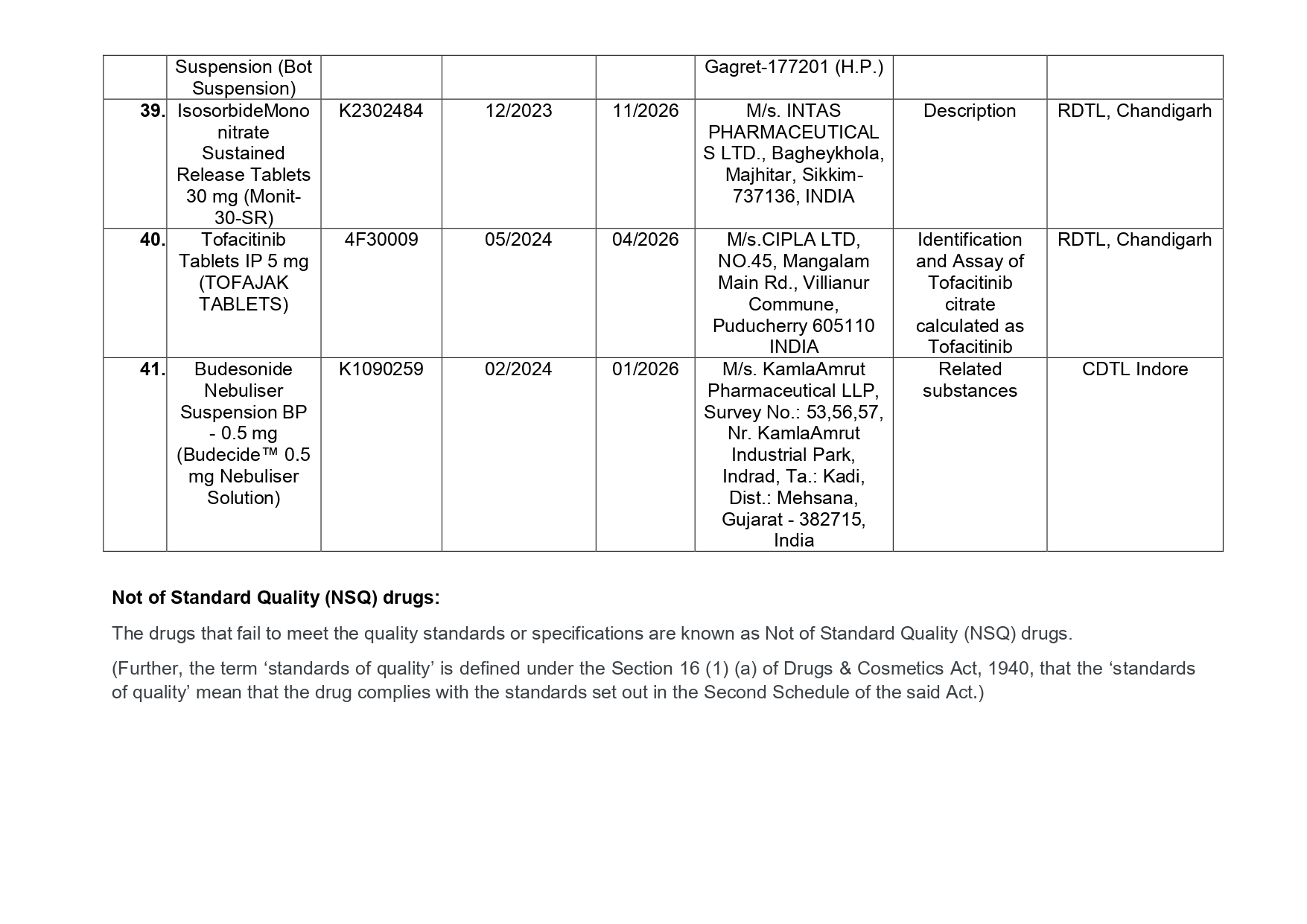
As per routine regulatory surveillance activity, the list of not of standard quality and spurious drugs is displayed on the CDSCO portal every month. “Identification of drug samples as NSQ is done based on the failure of the drug sample in one or the other specified quality parameters. The failure is specific to the drug products of the batch tested by the Government Laboratory and it does not warrant any concerns on the other drug products available in the market,” the ministry said.
Further, in the month of November, 2 drug samples have been identified as spurious drugs. Out of 2 drugs, one drug (Pantoprazole Gastro-Resistant Tablets I.P. (PAN-40) was picked by Bihar Drugs Control Authority which is mainly used for stomach and esophagus-related issues. Another drug (Amoxycillin and Potassium Clavulanate Tablets IP (AUGMENTIN 625 DUO) used in the treatment of bacterial infections was picked by CDSCO (NZ), Ghaziabad.
“These two drugs have been made by unauthorized and unknown manufacturers, using brand names owned by other companies. Investigation has been initiated in the matter,” the ministry said. The ministry said that this month’s NSQ reporting has witnessed an increasing participation of States in reporting NSQs to the central database.
“Increased reporting of NSQs/spurious identifications from states to central databases will further help in improving availability of quality medicines in the country and beyond,” the ministry stated.



