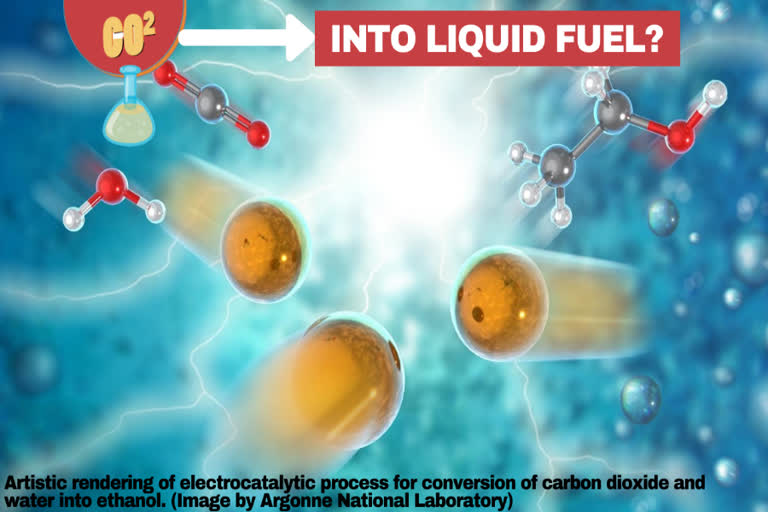
USA: Ethanol may be a particularly desirable commodity because it's an ingredient in nearly all U.S. gasoline and is widely used as an intermediate product within the chemical, pharmaceutical, and cosmetics industries.
"The process resulting from our catalyst would contribute to the circular carbon economy, which entails the reuse of CO2," said Di-Jia Liu, a senior chemist in Argonne's Chemical Sciences and Engineering division and UChicago CASE scientist within the Pritzker School of Molecular Engineering, University of Chicago.

The team's catalyst consists of atomically dispersed copper on carbon-powder support. By an electrochemical reaction, this catalyst breaks down CO2 and water molecules and selectively reassembles the broken molecules into ethanol under an external field. The electrocatalytic selectivity, or "Faradaic efficiency," of the method is over 90 percent, much above the other reported process.
Also Read: Can Artificial Intelligence(AI) be the solution to combat COVID-19
"With this research, we've discovered a replacement catalytic mechanism for converting CO2 and water into ethanol," said Tao Xu, a professor in chemistry and nanotechnology from Northern Illinois University. "The mechanism should also provide a foundation for the event of highly efficient electrocatalysts for CO2 conversion to a huge array of value-added chemicals."
However, consistent with Liu, "We could couple the electrochemical process of CO2-to-ethanol conversion using our catalyst to the electrical grid and cash in of the low-cost electricity available from renewable sources like solar and wind during off-peak hours." Because the method runs at coldness and pressure, it can start and stop rapidly in response to the intermittent supply of renewable electricity.
The team's research benefited from two DOE Office of Science User Facilities at Argonne -- the Advanced Photon Source (APS) and Center for Nanoscale Materials (CNM) -- also as Argonne's Laboratory Computing Resource Center (LCRC). "Thanks to the high photon flux of the X-ray beams at the APS, we've captured the structural changes of the catalyst during the electrochemical reaction,'' said Tao Li, a professor within the Department of Chemistry and Biochemistry at Northern Illinois University and an assistant scientist in Argonne's X-ray Science division.
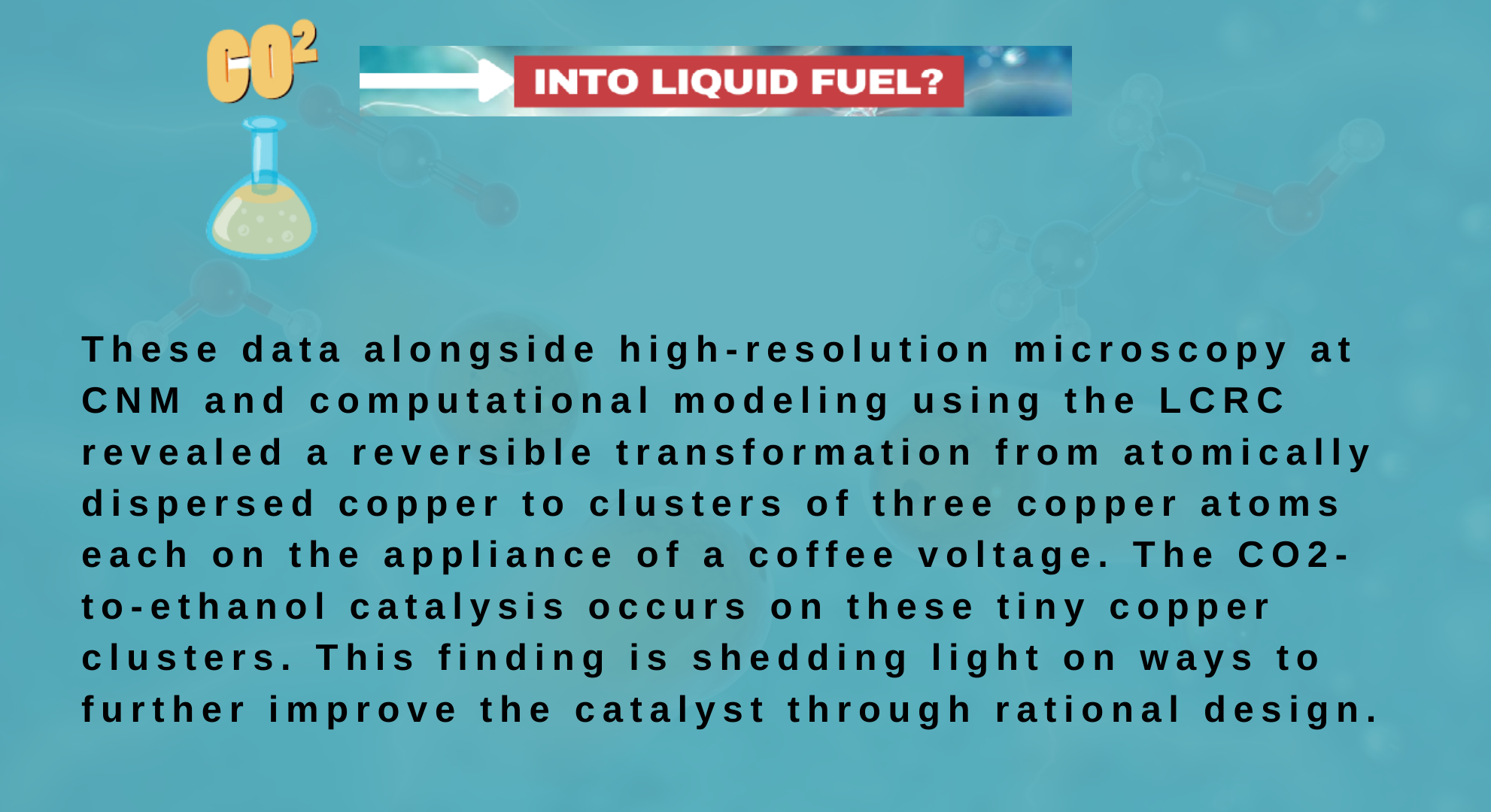
"We have prepared several new catalysts using this approach and found that they are all highly efficient in converting CO2 to other hydrocarbons," said Liu.
Also Read: Twitter allows iOS users to limit replies to their tweets