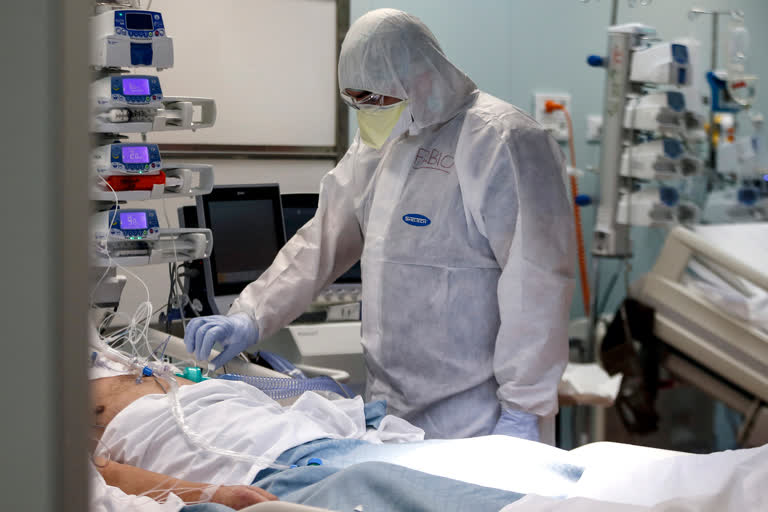
Houston: Three Indian Americans, who were hospitalised in a critical condition for COVID-19 here, are showing signs of recovery after being transfused with plasma from recovered coronavirus patients, hospital sources said.
As the vaccine for COVID-19 is not expected for months and new cases are increasing daily, doctors in Texas and around the country are experimenting with a new treatment based on an old technique, but aren't sure if it to be fully effective.
Read also: Signs missed, steps slowed in Trump's pandemic response
The treatment injects antibody-rich plasma from people who have recovered from the novel coronavirus into people who have severe cases of COVID-19, the disease caused by the virus. Antibodies are proteins in the blood that fight specific bacteria and viruses.
In the absence of a vaccine, doctors and scientists are looking to convalescent plasma because they consider it low risk and because it has been effective during past epidemics.
Read also: World's militaries face a new enemy in virus outbreak
Five patients at Baylor St. Luke's Medical Centre in Houston part of the Baylor College of Medicine have been treated with convalescent plasma, said Dr. Ashok Balasubramanyam, vice president of academic integration and associate dean of academic affairs at the Baylor College of Medicine.
The school has also been authorised to conduct a clinical trial, expected to start within a couple of weeks.
Three Indian American COVID-19 patients -- IT professional Rohan Bavadekar, Dr. Lavanga Veluswamy and Sushm Singh, are being treated at St Luke's Medical Center in Houston, and have found recently recovered donors with same blood groups for plasma transfusion.
According to hospital sources, they are showing positive signs of recovery and waiting for more donors for new rounds of plasma transfusion.
"Vaccines for broad use would take about 12-18 months, and we don't have time to wait," said Lola Adepoju, a health services researcher at the University of Houston College of Medicine. "...while those vaccines are being developed, what can we do? (Convalescent plasma) therapy definitely is one of those things we can actually pursue, said the researcher.
The Food and Drug Administration (FDA) is yet to approve the treatment but is allowing initial clinical trials. Because those trials are limited, doctors nationally can also request for the federal health agency's permission to use the treatment for severe COVID-19 cases.
Last week, the FDA tapped the Mayo Clinic to lead and coordinate the effort and evaluate the treatment's effectiveness.
Since then, hospital systems around the nation have registered through the Mayo Clinic to start treating patients with convalescent plasma.
On Thursday, a patient in Austin received a convalescent plasma infusion, the first in the city, said Dr Jeff Yorio, a hematologist and oncologist at Texas Oncology who helped get the programme started in Austin.
"Of course we all want to be very hopeful about a treatment like this, Yorio said. But at the same time, we don't truly know how effective it's going to be compared to other types of things we're already doing.
Yorio said that the programme has identified at least five other Austin patients with severe or life-threatening cases of COVID-19 whom doctors want to treat with plasma. "The FDA has approved patients within hours of receiving a request," he added.
"We're identifying the absolute sickest patients first," Yorio said. "If we're able to get more plasma out there that's available, then maybe we'd be able to expand that further to other patients."
The plasma came from Austin's blood bank, We Are Blood, which has connected with more than 20 potential plasma donors who have recovered from COVID-19 and that number should grow as word about the program gets out, said Nick Canedo, a spokesman for the blood bank.
Donors for convalescent plasma must have received a lab-confirmed positive test for the coronavirus or test positive for COVID-19 antibodies after recovery and be symptom-free for at least 28 days before donating, Canedo said, adding that many potential donors have been unable to get tested for the coronavirus because their symptoms were not severe enough to qualify for one.
Someone who initially tests positive and then has a second test for the coronavirus that comes back negative can donate after being symptom-free for 14 days, Canedo said.
Plasma recipients must be of the same blood type group as the donor.
This is not the first time physicians have used plasma to combat infectious diseases before a vaccine is developed. The technique was used to treat hemorrhagic fever in 1979 and Spanish influenza in 1918, Adepoju said, and it helped reduce mortality in both epidemics.
But when convalescent plasma was used to treat Ebola in 2014 in Guinea, Sierra Leone and Liberia, the research didn't find it to be effective.
With the FDA's blessing, doctors have also been trying to treat COVID-19 patients with hydroxychloroquine, a drug typically used to treat malaria and lupus that has shown in limited research potential to speed recovery.
But it comes with risks: Administered in high doses, the drug can cause heart arrhythmia and cardiac arrest, said Dr Kristin Mondy, chief of the division of infectious disease at the University of Texas at Austin's Dell Medical School.
"Hydroxychloroquine has bigger safety concerns than convalescent plasma," Mondy said.
PTI