New Delhi: Union Minister of Health & Family Welfare Dr Harsh Vardhan announced the successful completion of PAN-India 1000 Genome sequencing of SARS- CoV-2 on Saturday. He took a meeting with the Department of Biotechnology (DBT) and reviewed the COVID-19 activities of DBT, Biotechnology Industry Research Assistance Council (BIRAC) and DBT-Autonomous Institutions (AIs).
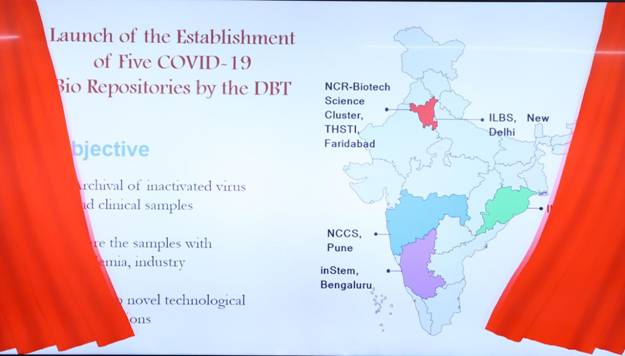
During the meeting, Dr Harsh Vardhan also launched and dedicated to the nation the largest network of five dedicated COVID-19 Biorepositories established by Department of Biotechnology in record time. These are at Translational Health Science and Technology Institute (THSTI) Faridabad, Institute of Life Science (ILS) Bhubaneshwar, Institute of Liver and Biliary Sciences (ILBS) New Delhi, National Centre for Cell Science (NCCS) Pune and Institute for Stem Cell Science and Regenerative Medicine (InStem) Bangalore. He complimented the efforts of DBT in “the relentless war for mitigation of this Pandemic”.
Dr Harsh Vardhan said, “Given the importance of this information for public health response initiatives requiring investigation into the transmission of COVID-19, the sequence data will soon be released in Global Initiative on Sharing All Influenza Data (GISAID) for use by researchers across the Globe”.
“The information in the database will improve our understanding on how the virus is spreading, ultimately helping to interrupt the transmission chains, prevent new cases of infection, and provide impetus to research on intervention measures”, he added.
Read: Abrogation of Article 370 from J&K has long term vision, say experts
The Minister also pointed out, “The data analysis, which is ongoing, may bring out some interesting conclusions to help in our fight against COVID-19.”
Dr Harsh Vardhan also highlighted that “16 Vaccine Candidates are in different stages of development. The BCG Vaccine is undergoing phase 3 trial, Zydus Cadila DNA Vaccine is in phase I / II trial and 4 Vaccine candidates are in advanced stages of pre-clinical study”. “5 Good clinical laboratory practice (GCLP) clinical trial sites have been developed and 6 animal models for Vaccine Development Studies are also ready”, he said.
The Department of Biotechnology had launched a Pan India 1000 SARS--CoV-2 RNA Genome Sequencing programme in May this year to be done by Autonomous Institutes of DBT, collaborating with national laboratories and clinical organizations.
The Consortium has achieved its initial goal of completing the sequencing of 1000 SARS-CoV-2 genomes from nasopharyngeal and oropharyngeal swabs collected from individuals testing positive for COVID19 by Real-Time PCR. The samples were collected across 10 states covering different zones within India.
Read: SKorean sect leader held for obstructing anti-pandemic efforts
DBT is supporting COVID-19 Bio Repositories through a well-strategized plan so that novel technological interventions can be developed in due course of time. The main purpose of these biorepositories is archival of inactivated virus and clinical samples, including naso-oropharyngeal swabs, stool, urine, saliva, serum, plasma, PBMC and Serum.
These designated biorepositories will use the clinical samples for R&D purpose and are authorized to share the samples with academia, industry and commercial entities involved in the development of diagnostics, therapeutics, vaccines etc., after scrutinising the purpose of the request and ensuring benefit to the country. Standard Operating Procedures (SoPs) for sample collection, transportation, aliquoting, storage and sharing have been developed. As on date, 44452 clinical samples have been collected and stored in these five centres. More than 5,000 samples have been shared.
During the meeting which was attended by Dr Renu Swarup Secretary DBT, and joined through video-links by Senior officers of DBT and it is Autonomous Institutes and Public Sectors BIRAC and BIBCOL, the Minister has presented an update on the DBT–BIRAC COVID 19 Research Consortia under which more than 150 Research Groups have been supported involving nearly 80 Industry /Academia collaborations, 40 Academic Research Institutes and more than 25 Startup Research Groups.
The consortium has successfully developed 100 per cent self-reliance for producing more than 5 lakh RTPCR diagnostic kits per day. 4 technologies of DBT AI's have been transferred to the Industry for commercial manufacturing of diagnostic kits. DBT AI's are also providing services for Diagnostic Testing, Kit Validation and Antiviral testing.
Also Read: Man accused of carrying beef attacked in Gurugram



