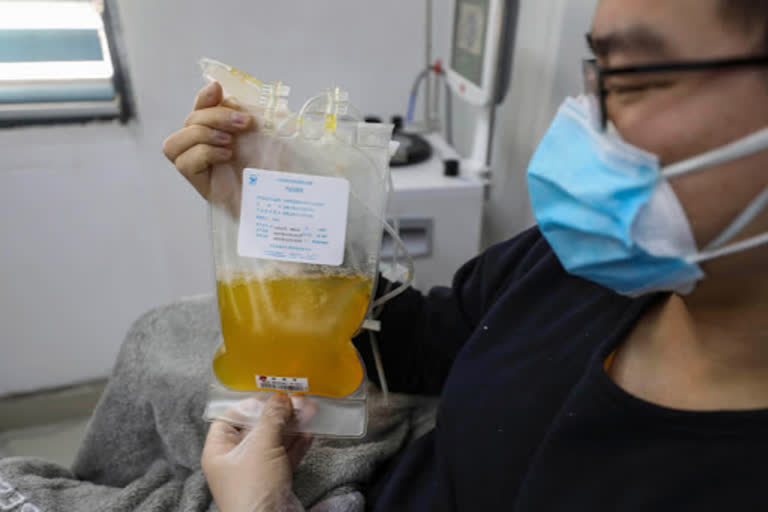
Hyderabad: The US Food and Drug Administration has officially allowed Johns Hopkins University researchers to conduct clinical trials to test a therapy for COVID-19. The therapy will use plasma from recovering patients.
According to a release, this will allow the university to test the use of blood plasma to boost the immune systems of health-care providers, first responders and others who are at high risk of contracting the disease.
Arturo Casadevall, a Johns Hopkins infectious disease expert, believes that this could help in treating critically ill COVID-19 patients and will also be helpful in boosting the immune systems of health care providers.
This required a team of physicians and scientists from around the United States. After which a network of hospitals and blood banks were established that will collect, isolate, and process blood plasma from COVID-19 survivors.
"The ability to carry out a prophylaxis trial will tell us whether plasma is effective in protecting our health care workers and first responders from COVID-19," Casadevall said.
Using blood products from people who’ve already beaten a disease is a long-established approach. After the FDA's approval, the researchers will be able to begin testing the technique's effectiveness in boosting the immune systems of health care workers and others at high risk of exposure to the disease.
As we all know that coronavirus is a new disease and there is no effective vaccines for treating the infection. Casadevall and his team abide by the fact that using plasma from the recovered patients could provide immediate immunity to the most at-risk individuals.
Also Read: Will COVID-19 crisis be a litmus test for President Trump