New Delhi: In a strong endorsement of Indian scientists and doctors, Prime Minister Narendra Modi Monday morning received the first dose of COVAXIN at All India Institute of Medical Sciences (AIIMS) in New Delhi. COVAXIN has been developed by Hyderabad based vaccine manufacturer Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV) and contains an inactivated virus incapable of causing the disease.
The Prime Minister received the vaccine six weeks after its roll out on January 16, when the country started vaccinating healthcare professionals and other frontline workers, including security personnel.

“Took my first dose of the COVID-19 vaccine at AIIMS,” Prime Minister Narendra Modi announced in a tweet early Monday morning.
Praising the doctors and scientists for the quick development of the vaccine, the Prime Minister said: “Remarkable how our doctors and scientists have worked in quick time to strengthen the global fight against COVID-19.”
Prime Minister’s office said Sister P Niveda, from Puducherry, administered COVAXIN (Bharat BioTech) to Prime Minister Modi.
“I appeal to all those who are eligible to take the vaccine. Together, let us make India COVID-19 free!,” Prime Minister Modi said in his appeal to the countrymen.
In January this year, India’s drug regulator, the Drugs Controller General of India, authorised two vaccines for emergency use against the Covid-19 (SarS-CoV-2) virus which has killed more than 2.5 million people worldwide and more than 1,57,000 people in the country.
The emergency use authorisation was granted to the CoviShield vaccine developed by Oxford University and AstraZeneca, which is being produced in the country by the world’s largest vaccine producer, Pune based Serum Institute of India and India’s indigenous COVAXIN developed jointly by Hyderabad based Bharat Biotech and the Indian Council of Medical Research (ICMR-NIV).
READ: PM Modi takes first dose of Bharat BioTech's COVAXIN at AIIMS
PM Modi takes indigenous COVAXIN
Prime Minister Narendra Modi delivered two important messages in taking the Covid jab as he received the indigenously developed COVAXIN (Bharat Biotech-ICMR-National Institute of Virology) rather than taking Covishield developed by Oxford and AstraZeneca, which is a strong endorsement of capabilities of Indian scientists by the highest political executive of the country.
Secondly, it is also important as the country’s drug regulator, the DCGI, has authorised the emergency use of COVAXIN before the completion of the third phase of the vaccine trial in the country, which had come under criticism.
Prime Minister Narendra Modi’s decision to take COVAXIN rather than COVISHIELD sends a strong message to the skeptics that questioned the drug regulator’s decision to authorise emergency use of locally developed COVAXIN.
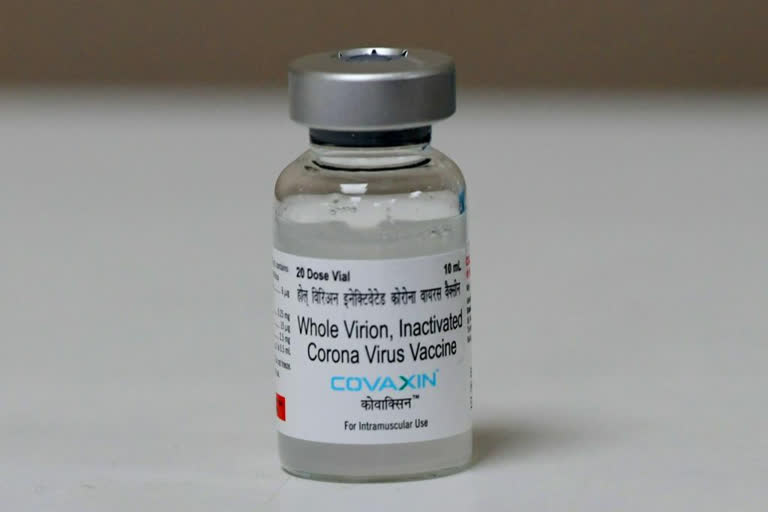
According to the vaccine developer, COVAXIN is being developed using the whole-virion inactivated Vero cell-derived platform technology which does not replicate and, as such, is unlikely to revert and cause pathological effects.
These kinds of vaccines contain dead viruses, incapable of infecting people but still able to instruct the immune system to mount a defensive reaction against an infection.
READ: Vaccination phase 3: How to register, choose CVC, all you need to know
PM waits for mass rollout of vaccine
Prime Minister Narendra Modi also faced strong criticism for not taking the vaccine himself at the start of the roll out in January this year.
The decision was a departure from the policies followed in some other countries, including the US, where the then Vice President Mike Pence and the country's President-elect Joe Biden were administered the Covid vaccine in December last year during the initial days of vaccine roll out to build people’s confidence, which was considered crucial for overcoming vaccine hesitancy among the masses.
In India, Prime Minister Modi consciously followed a policy to wait for the day when the second phase of the vaccination drive is rolled out, allowing senior citizens and people above the age of 45 years with co-morbidities to register on the CoWin portal.
In the first phase, the vaccine is being administered to India’s healthcare workers, including doctors, nurses, paramedics and sanitation staff working in government and private hospitals and security personnel who have been at the forefront of the country’s war against the deadly virus.
More than 14 million healthcare workers and security personnel have so far received the Covid vaccines in the first phase.
READ: Registration for next phase of COVID-19 vaccination to open at 9 am on Monday