Hyderabad: Pfizer India has become the first pharmaceutical firm to seek from the Drugs Controller General of India (DCGI) an emergency use authorisation for its COVID-19 vaccine in the country.
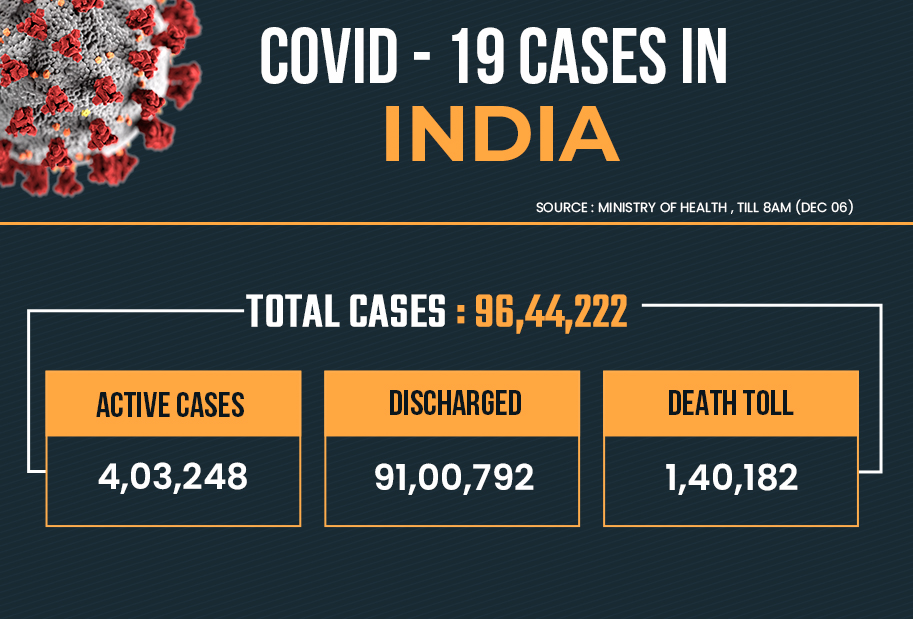
This is the first such request received by the DGCI amid the race to find a vaccine for the deadly virus, which has affected over 96 lakh people in India.
The firm, in its application submitted to the drug regulator, has sought permission to import the vaccine for sale and distribution in the country, besides waiver of clinical trials on Indian population in accordance with the special provisions under the New Drugs and Clinical Trials Rules, 2019, official sources said.
It is pertinent to mention here that five vaccines are in advanced phases of clinical trials in India with the Serum Institute of India conducting the phase-3 trial of the Oxford-Astrazeneca COVID-19 vaccine, while the indigenously developed vaccine by Bharat Biotech in collaboration with ICMR has already started the phase-3 clinical trial.
- Delhi
New Delhi: Nearly 11% of those in the national capital who tested negative for COVID-19 in rapid antigen tests (RAT) but had symptoms for the disease were found to have been afflicted by the viral infection in RT-PCR test between September 1 and November 7, according to official data.
According to the data shared by health authorities in response to an RTI query, out of the 56,862 symptomatic patients who tested negative in rapid antigen tests, 32,903 were retested through RT-PCR and of them 3,524 were found COVID-19 positive.
- Maharashtra
Pune: A total of 17 volunteers have been administered Russia's Sputnik V coronavirus vaccine at a Pune hospital as part of the human clinical trials, doctors said on Sunday.
Sputnik V vaccine has been developed by the Gamaleya National Research Center of Epidemiology and Microbiology and Russian Direct Investment Fund (RDIF).
As per reports, India has purchased 100 million doses of the candidate from Russia.
- Punjab
Chandigarh: Chief Minister Amarinder Singh has written to Prime Minister Narendra Modi, seeking priority allocation of COVID-19 vaccine to the state, on account of its higher mortality rate resulting from the population age profile and high levels of co-morbidities.
Pointing out that the vaccines currently under consideration would not reduce transmission so much as prevent serious disease, Singh said the best use of these vaccines would therefore be in preventing serious illness in the most susceptible groups, including elderly and persons with high morbidity.
- Tamil Nadu
Chennai: Congress Committee President KS Alagiri has tested positive for coronavirus on Sunday and is admitted to a private hospital.
"TNCC President KS Alagiri has tested positive for coronavirus following a test today morning and has been admitted to a private hospital for treatment," A Gopanna, TNCC media department chairman said in a statement.
Gopanna asked those who had come in contact with Alagiri to get tested. Alagiri attended a meeting with Congress and DMK members on Thursday.
- Gujarat
Ahmedabad: Chief Minister Vijay Rupani on Sunday said that the state will administer the COVID-19 vaccines in four stages i.e. to healthcare workers, corona warriors, people above 50 years and then people below 50 years with comorbidities.
The COVID-19 vaccination in Gujarat would start with around 3.96 lakh healthcare workers, official sources said.



