Unites States: "One of the key features of breast cancer is that most patients survive if the disease stays local, but there is a greater than 70% drop in survival if the cells have metastasized," said Luis Solorio, a Purdue assistant professor of engineering, who co-led the research team. "However, once the cells leave the primary tumor, they are often no longer responsive to the drugs that initially worked for the patient. We wanted to develop a system that could help us better understand how the physiology of a new tissue space affected tumor cells upon invasion into the new organ."
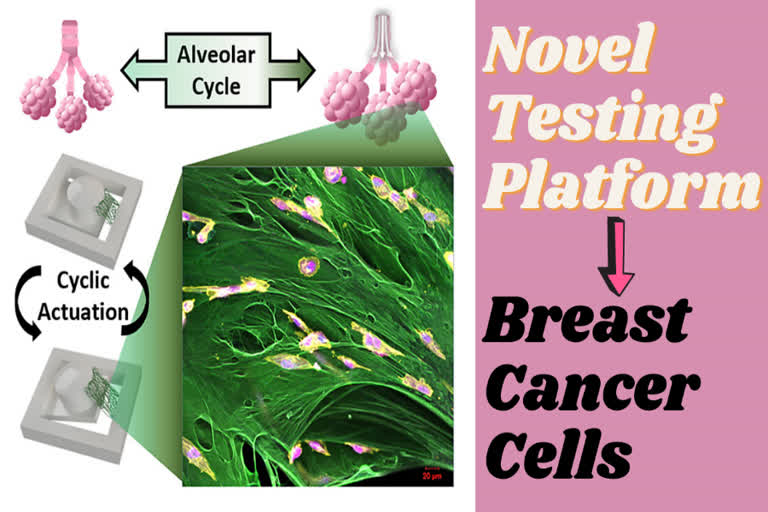
- The Purdue researchers created a magnetically moving cell culturing system where the cancer cells can be grown in 3D on a suspended extracellular matrix protein that is abundant in early metastatic lung tissue in order to evaluate the impact of mechanical forces.
- They were able to incorporate the strain amplitude and rate of breathing in this tissue mimic. The researchers found that the cells quit dividing under these conditions. The research is published in Advanced Functional Materials.
"Never before has the concept of motion been interrogated as a component of the tumor microenvironment," said Michael Wendt, a Purdue associate professor of medicinal chemistry and molecular pharmacology. "We now understand that healthy organs utilize motion to resist metastatic colonization. The development of this microactuator system will not only continue to yield increased biological understanding, of metastasis, but it will also serve as a platform for us to better evaluate pharmacological inhibitors of the most lethal aspect of cancer progression."
Hyowon "Hugh" Lee, an associate professor of engineering and a researcher at the Birck Nanotechnology Center, co-led the research team.
"This is the first attempt to engineer a cell culture system that can apply mechanical forces on a suspended tissue," Lee said. "Most bioreactors with mechanical stimulation capabilities rely on growing 2D cell culture on flat non-biological substrates, but we are using a custom magnetic actuator and suspending a layer of fibronectin to grow 3D cancer cells like a miniature tissue. "Our system better mimics the physiological environment without using artificial substrates. Using this platform, we show that certain cancer cells slow down their proliferation due to the cyclic stretching of breathing."