London: Scientists at Oxford University said that their experimental coronavirus vaccine has been shown in an early trial to prompt a protective immune response in hundreds of people who got the shot.
British researchers first began testing the vaccine in April in about 1,000 people, half of whom got the experimental vaccine. Such early trials are usually designed only to evaluate the safety, but in this case, experts were also looking to see what kind of immune response was provoked.
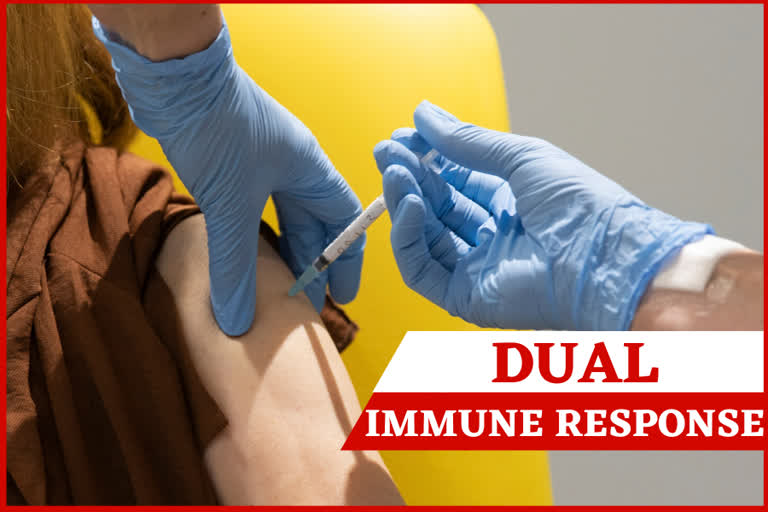
"We are seeing good immune response in almost everybody," said Dr Adrian Hill, director of the Jenner Institute at Oxford University. "What this vaccine does particularly well is to trigger both arms of the immune system," he said.
Hill said that neutralising antibodies are produced — molecules that are key to blocking infection. In addition, the vaccine also causes a reaction in the body's T-cells which help to fight off the coronavirus.
Read also:UK firm declares positive results from COVID-19 protein treatment
He said that larger trials evaluating the vaccine's effectiveness, involving about 10,000 people in the UK as well as participants in South Africa and Brazil are still underway. Another big trial is slated to start in the US soon, aiming to enroll about 30,000 people.
How quickly scientists are able to determine the vaccine's effectiveness will depend largely on how much more transmission there is, but Hill estimated they might have sufficient data by the end of the year to decide if the vaccine should be adopted for mass vaccination campaigns.