Amsterdam (The Netherlands): Health technology company Royal Philips on Tuesday announced that it has received clearance from the US Food and Drug Administration (FDA) for its wearable biosensor, that can be used to monitor COVID-19 patients in hospitals.
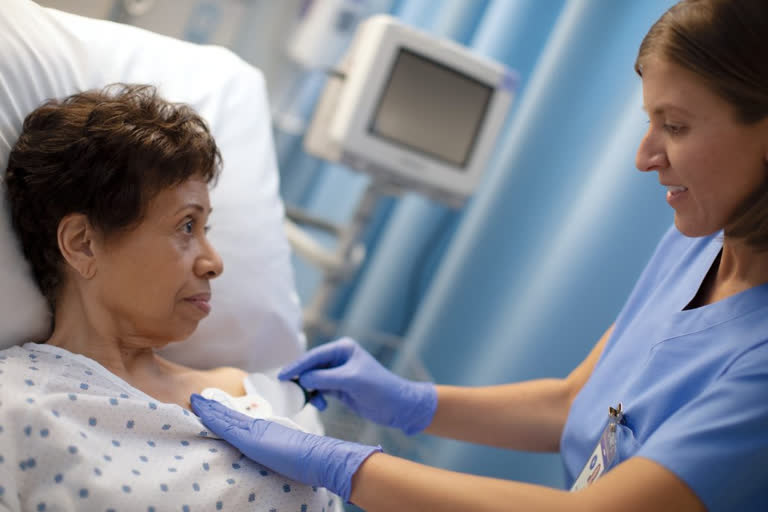
The Philips Biosensor BX100 is a lightweight, disposable biosensor which can be used to monitor multiple patients across multiple rooms in hospitals.
The biosensor is in fact a five-day single-use patch and can be attached discreetly to the chest of the patient to collect, store, measure and transmit respiratory rate and heart rate every minute.
Besides the above, the patch can also be used to monitor the patient's posture, activity level, and ambulation.
"During this unprecedented time of COVID-19, the Philips Biosensor BX100 helps provide rapid deployment for clinical surveillance to help decrease risk of exposure of healthcare workers while acquiring frequent patient vitals, and easing the demand for personal protective equipment (PPE)," Peter Ziese, General Manager Monitoring and Analytics at Philips was quoted by an official release.