Washington: U.S. regulators have approved the first long-acting drug combo for HIV, monthly shots that can replace the daily pills now used to control infection with the AIDS virus.
“That will enhance the quality of life” to need treatment just once a month, said Dr Steven Deeks, an HIV specialist at the University of California, San Francisco, who has no ties to the drug’s makers. “People don’t want those daily reminders that they’re HIV infected.”
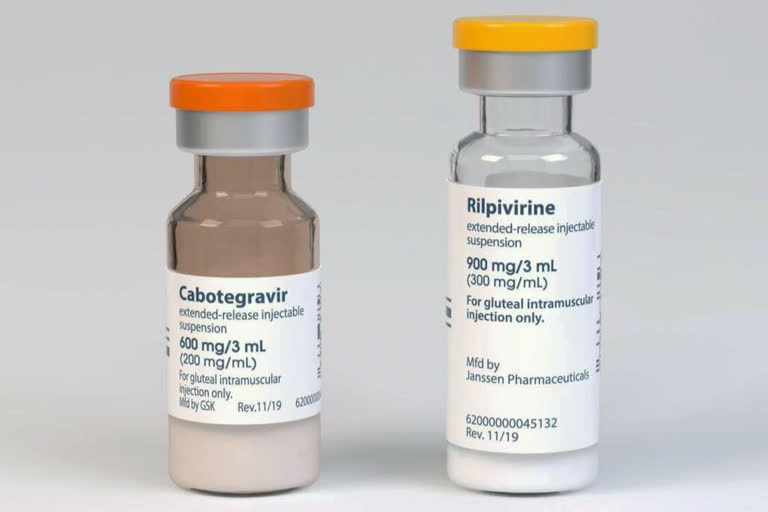
Cabenuva combines rilpivirine, sold as Edurant by Johnson & Johnson’s Janssen unit, and a new drug — cabotegravir, from ViiV Healthcare. They’re packaged together and given as separate shots once a month. Dosing every two months also is being tested.
The U.S. Food and Drug Administration approved Cabenuva for use in adults who have had their disease well controlled by conventional HIV medicines and who have not shown signs of viral resistance to the two drugs in Cabenuva.
Read:|WHO cautions over delayed HIV response measures owing to COVID-19
The agency also approved a pill version of cabotegravir to be taken with rilpivarine for a month before switching to the shots to be sure the drugs are well tolerated.
ViiV said the shot combo would cost $5,940 for an initial, higher dose and $3,960 per month afterwards. The company said that is “within the range” of what one-a-day pill combos cost now. How much a patient pays depends on insurance, income and other things.