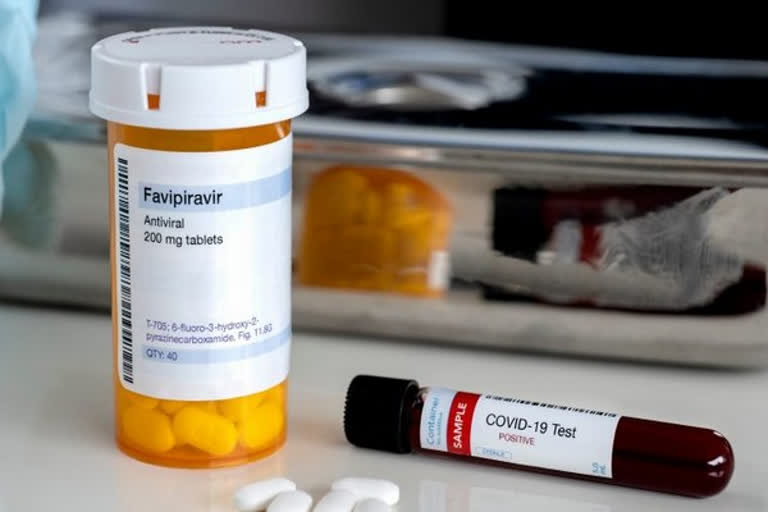
New Delhi: Glenmark Pharmaceuticals on Thursday said it has become the first company in India to receive approval from Drug Controller General of India (DCGI) to conduct clinical trials of Favipiravir antiviral tablets for the treatment of COVID-19 patients.
Having internally developed the active pharmaceutical ingredients (API) and the formulations for the product, the company filed the product for clinical trials with the DCGI and has received approval for conducting the trial on mild to moderate patients, Glenmark Pharmaceuticals said in a statement.
The Mumbai-based company is the first pharmaceutical company in India to be given an approval by the regulator to start the trial on COVID-19 patients in the country, it added.
Favipiravir has demonstrated activity against influenza viruses and has been approved in Japan for the treatment of novel influenza virus infections, it added.
As per the clinical trial protocol approved, 150 subjects with mild to moderate COVID-19 will be randomised in the study in a 1:1 ratio to Favipiravir with standard supportive care or standalone standard supportive care, the company said.
Read more:India needs Rs 65,000 cr to feed poor during pandemic, can afford it: Rajan