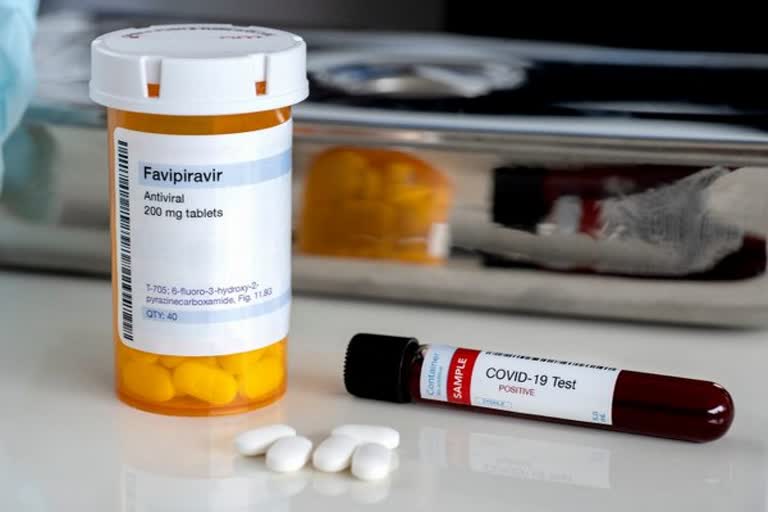
New Delhi: Drug firm Glenmark Pharmaceuticals on Saturday said it has launched antiviral drug Favipiravir, under the brand name FabiFlu, for the treatment of patients with mild to moderate COVID-19 at a price of about Rs 103 per tablet.
The drug will be available as a 200 mg tablet at a maximum retail price (MRP)of Rs 3,500 for a strip of 34 tablets, Glenmark Pharmaceuticals said.
FabiFlu is the first oral favipiravir-approved medication in India for the treatment of COVID-19, it said in a statement.
It is a prescription-based medication, with recommended dose being 1,800 mg twice daily on day one, followed by 800 mg twice daily up to day 14, it added.
The tablets are being produced by the company at its Baddi facility in Himachal Pradesh. The drug will be available both through hospitals and the retail channel, Glenmark said.
The Mumbai-based firm had on Friday received the manufacturing and marketing approval from the Drugs Controller General of India (DCGI).
"This approval comes at a time when cases in India are spiralling like never before, putting a tremendous pressure on our healthcare system," Glenmark Pharmaceuticals Chairman and MD Glenn Saldanha said in the statement.
The company hopes that the availability of an effective treatment such as FabiFlu will considerably help assuage this pressure, and offer patients in India a much needed and timely therapy option, he added.
"Glenmark will work closely with the government and medical community to make FabiFlu quickly accessible to patients across the country," he added.
The company has successfully developed the active pharmaceutical ingredient (API) and the formulation for FabiFlu through its in-house research and development team, Glenmark said.
Read more:Boycotting Chinese goods will not hurt China's economy: Chidambaram
"We chose to initiate work on Favipiravir, as it has proven in-vitro activity against SARS CoV2 virus, which is the virus responsible for COVID-19.
"Second is it has a wide therapeutic safety margin for COVID-19 at the dose that we administer,"Glenmark Pharmaceuticals President India Formulations, Middle East and AfricaSujesh Vasudevan said at an online press conference.
Moreover, it is an oral product and that is a huge benefit especially when the hospital infrastructure is under strain, he added.
Manufacturing and marketing approval has been granted as part of an accelerated approval process, considering the emergency situation of the COVID-19 outbreak in India, Glenmark said.
The approval's restricted use entails responsible medication usage where every patient must have signed informed consent before treatment initiation, it added.
Favipiravir can be used for coronavirus patients with co-morbid conditions such as diabetes and heart disease with mild to moderate COVID-19 symptoms,Glenmark said.
It offers rapid reduction in viral load within four days and provides faster symptomatic and radiological improvement.
Favipiravir has shown clinical improvement of up to 88 per cent in mild to moderate COVID-19 cases, it said.
Favipiravir has been approved in Japan since 2014 for the treatment of novel or re-emerging influenza virus infections.
Last month, Glenmark also announced that it is conducting another clinical trial to evaluate the efficacy of two antivirals Favipiravir and Umifenovir as a combination therapy in moderate hospitalised adult COVID-19 patients in India.
India on Saturday saw another record spike of 14,516 new COVID-19 cases in a single day, pushing the tally to 3,95,048, while the death toll rose to 12,948 with 375 new fatalities, according to Union Health Ministry data.
(PTI Report)