New Delhi: Giving a much-needed impetus to the global fight against tuberculosis (TB), the World Health Organization (WHO) has endorsed three rapid molecular Truenat assays being developed jointly by Indian Council of Medical Research (ICMR), Foundation for Innovative New Diagnostics (FIND), and Molbio Diagnostics.
The assays will be used for initial diagnosis of tuberculosis (TB) and subsequent detection of rifampicin resistance in adults and children with signs and symptoms of pulmonary TB.
Both Molbio Diagnostics and FIND work for innovations in the global health care sector.
Truenat assays have now been incorporated into the India National TB Elimination Programme (NTEP) after a recommendation from ICMR, India’s apex medical research institute.
“This is a matter of pride for ICMR, Department of Health Research (DHR). It was a long journey to advance indigenous diagnostic technologies for diagnosis of TB and MDR/XDR-TB. Truenat is already accepted for use under the NTEP in India.
Endorsement of Truenat by WHO will enable other low-and middle-income countries to procure Truenat for diagnosis of TB and rifampicin resistance, thus supporting TB elimination in developing countries,” said Dr Balram Bhargava, Secretary DHR and Director General ICMR on Friday.
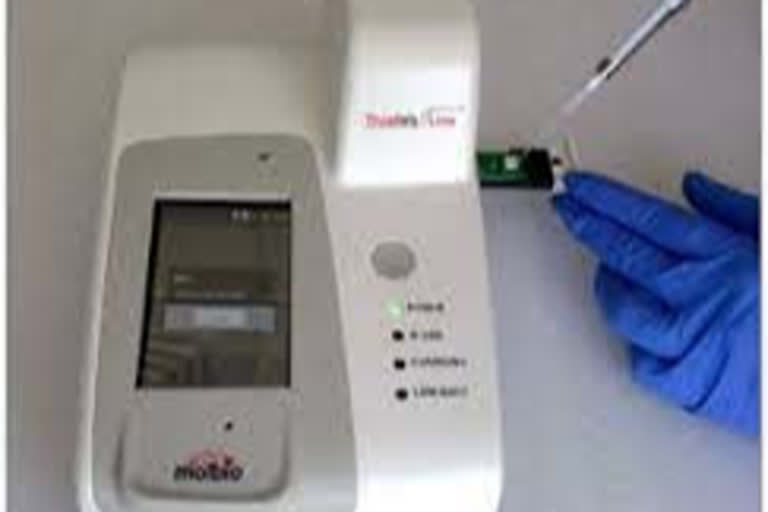
Officials in ICMR said that Truenat MTB and Truenat MTB Plus detect mycobacterium tuberculosis bacteria for TB diagnosis, while Truenat MTB-RIF Dxidentifies resistance to rifampicin, the most commonly used first-line treatment.
“All three tests are run on the portable, battery-operated Truenat device and provide results in less than an hour,” officials said.
The development could also inspire the medical practitioners and scientists across India to make the country TB free. The government of India also vouches to make the country TB free by 2025.
TB remains the leading cause of death from an infectious disease worldwide, with around 10 million cases and 1.5 million deaths in 2018.
Drug-resistant TB poses a particular challenge, with growing resistance to rifampicin and other drugs that treat the disease. In 2018, around half-a-million new cases of rifampicin-resistant TB were diagnosed.
Urgent action is needed to close the gap in TB diagnosis and treatment, particularly in low-resource settings, to reach the WHO target of ending TB by 2030. To that end, bringing sensitive TB diagnosis and drug susceptibility testing closer to patients is a key priority for global TB control, but requires robust point-of-care diagnostic tests that are easily implementable at lower levels of the healthcare system.
Officials said that the Truenat tests use real-time micro polymerase chain reaction (PCR) technology. Truenat devices function in a wide range of environmental conditions with minimal user input, making them suitable for use in primary healthcare settings that typically have fewer facilities than reference laboratories in which rapid molecular tests are usually conducted.
Truenat was developed by Bigtec Labs, the R&D subsidiary of Molbio Diagnostics. ICMR conducted multicentre validation of Truenat MTB and Truenat MTB-RIF Dxassays followed by a feasibility study under the national programme and found them to be on par with the internationally recognized Xpert MTB/RIF molecular assay (Cepheid, Sunnyvale, USA) in terms of sensitivity and specificity, and detection of rifampicin resistance.
ICMR officials said that the performance and accuracy of the Truenat assays have been assessed by FIND in a real-world multicentre diagnostic accuracy study conducted in India, Peru, Ethiopia and Papua New Guinea.
“The study determined the diagnostic accuracy of the Truenat tests when performed in peripheral laboratories, compared with culture as the reference standard as well as the internationally recognized Xpert MTB/RIF Ultra and Xpert MTB/RIF assays,” officials said.
Also read: 29 killed as train hits Sikh pilgrims bus in Pakistan