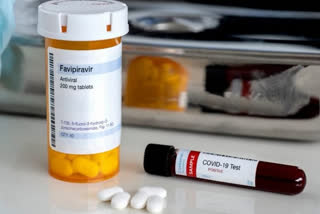
New Delhi: India's apex drug regulator, drug controller general of India (DCGI) has asked two domestic pharma giant for clinical proof as a requirement to manufacture Favipiravir 600mg and 800mg tablets.
Favipiravir tablets 200mg and 400mg are already approved for restricted emergency use for COVID-19 in India.
The two domestic pharma giant Cipla and Hetero in their meeting with DCGI officials on Tuesday had presented their proposal for Favipiravir 600mg and 800mg tablets before the subject expert committee.
"After detailed deliberations, the committee recommended that the firm should submit detailed clinical justification for the proposed strength of Favipirnavir alongside supportive literature and updated clinical data from other countries for further consideration," officials said.
The DCGI, in another development, has granted permission for phase IV clinical trials of Remdesivir 100mg injection vial after pharma company Jubilant Generics Limited submitted documents in support of the trial.