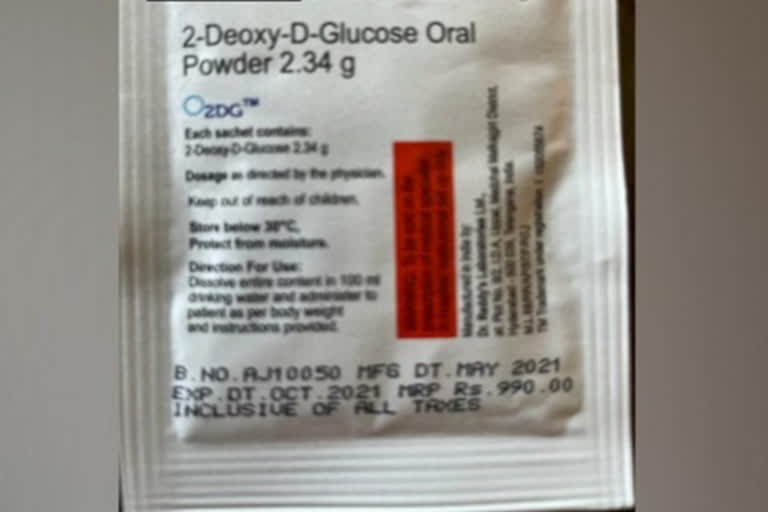
New Delhi: The Defence Research and Development Organisation's (DRDO) on Tuesday said that the anti-COVID drug 2DG is approved for emergency use as an adjunct therapy to the standard of care in the treatment of coronavirus patients in hospital settings.
"Ideally, 2DG should be prescribed as early as possible by doctors for moderate to severe COVID patients for a maximum duration of up to 10 days," it said.
DRDO further said that uncontrolled diabetes, severe cardiac problem, ARDS, severe hepatic and renal impairment patients have not been studied yet with 2DG, and hence caution should be exercised.
Read:|DRDO's anti-COVID drug priced at Rs 990
The 2DG should not be given to pregnant and lactating women and patients below 18 years.
"Patients and attendants are advised to request the hospital to contact Dr Reddy's lab Hyderabad for medicine supply at email: 2DG@drreddys.com," it added.
The first batch of DRDO's anti-COVID-19 drug was released on May 17 by Defence Minister Rajnath Singh and health minister Dr Harsh Vardhan, after the Drugs Controller General of India permitted the emergency use authorisation of 2-deoxy-D-glucose (2-DG), anti-viral drug as an adjunct therapy for moderate to severe coronavirus patients.