New Delhi: To fight against mpox, the World Health Organisation (WHO) has asked manufacturers of mpox in vitro diagnostics (IVDs) to submit an expression of interest for Emergency Use Listing (EUL). The global health watchdog has been undergoing discussions with manufacturers about the need for effective diagnostics, particularly in low-income settings.
Stating that testing is key for people to get treatment and care as early as possible and prevent further spread, since 2022, WHO has delivered around 150,000 diagnostic tests for mpox globally, of which over a quarter have gone to countries in the African Region. In the coming weeks, WHO will deliver another 30,000 tests to African countries.
“With as many as 1000 suspected cases reported in the Democratic Republic of the Congo alone this week, the demand for diagnostic tests is on the rise. In this heavily affected country, WHO has worked with partners to scale up diagnostic capacity to respond to the upsurge of cases.
Since May 2024, six additional labs have been equipped to diagnose mpox, enabling a decentralization of testing capacity from major cities to affected provinces. Two of these labs are in South Kivu, selected to respond to the outbreak of the new viral strain, called Ib. Thanks to these efforts, testing rates have dramatically improved in the country, with four times as many samples tested in 2024 so far as compared to 2023,” WHO said.
WHO has also updated its diagnostic testing guidance to detect the new virus strain and is working with countries to roll it out. Earlier, WHO issued target product profiles to guide manufacturers in the development of new diagnostic tests.
WHO Director-General Dr Tedros Adhanom Ghebreyesus declared on 14 August 2024 that the upsurge of mpox in the Democratic Republic of the Congo (DRC) and a growing number of countries in Africa constitutes a public health emergency of international concern (PHEIC) under the International Health Regulations (2005).
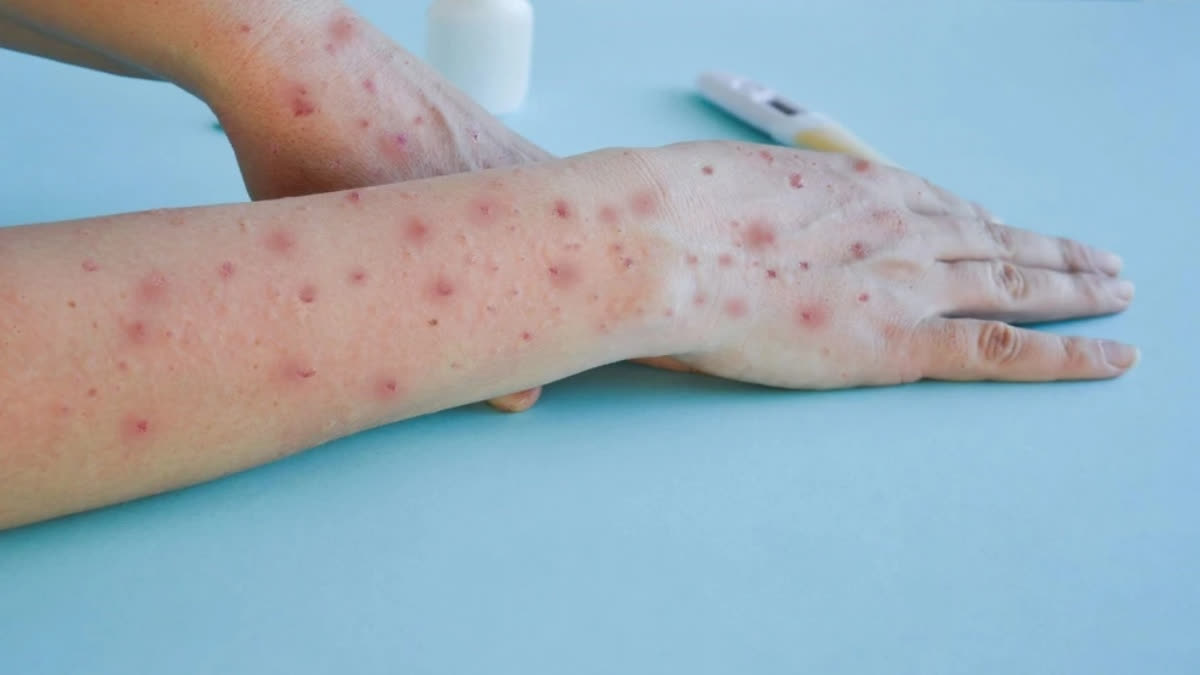
Manufacturers of IVDs are now asked to submit available quality, safety and performance data to WHO as soon as they can. IVDs are tests done in laboratories to detect a pathogen. Detection of viral DNA by PCR (Polymerase Chain Reaction) testing is the gold standard for mpox diagnosis.
It detects the virus's DNA in samples taken from skin lesions, such as fluid or crusts from vesicles or pustules. Testing of blood is not recommended for routine diagnosis and antibody detection methods may be used for retrospective case classification but not for diagnosis.
Through the EUL procedure, WHO can approve medical products such as vaccines, tests and treatments for use, evaluating the acceptability of using specific products for time-limited procurement in emergencies. The process aims to assist countries, which have not approved the medical products through national approval processes, to procure the critically needed products such as tests through UN agencies and other partners.
Mpox is an illness caused by the monkeypox virus, a species of the genus Orthopoxvirus, that can be transmitted to humans through contact with someone infectious, with contaminated materials, or with infected animals.
Read More:
- WHO Announces Limited Pauses in Gaza Fighting to Allow for Polio Vaccinations
- India Registers 1,11,807 Cases Of Mosquito-Borne Diseases This Year